Abstract
Nodular regenerative hyperplasia (NRH) is an uncommon liver condition characterized by diffuse transformation of the hepatic parenchyma into regenerative nodules without fibrosis. Portal vasculopathy caused by abnormal hepatic venous flow may induce hepatocyte hyperplasia, which forms regenerative nodules. Underlying diseases or certain drugs may also be the cause of NRH. This condition is often underdiagnosed as the patients remain asymptomatic until development of portal hypertension, and histopathologic confirmation by liver biopsy is the only way of making a definite diagnosis. The management mainly involves prevention and treatment of the complications of portal hypertension. The frequency of diagnosis of NRH has increased rapidly in recent years, however, only a few cases have been reported in Korea. Here, we report on a case of NRH of the liver combined with toxic hepatitis.
Go to : 
References
1. Wanless IR. Micronodular transformation (nodular regenerative hyperplasia) of the liver: a report of 64 cases among 2,500 au-topsies and a new classification of benign hepatocellular nodules. Hepatology. 1990; 11:787–797.
2. Steiner PE. Nodular regenerative hyperplasia of the liver. Am J Pathol. 1959; 35:943–953.
3. Nzeako UC, Goodman ZD, Ishak KG. Hepatocellular carcinoma and nodular regenerative hyperplasia: possible pathogenetic relationship. Am J Gastroenterol. 1996; 91:879–884.
4. Lee DH, Lee JI, Ko YT, Kim YW. Nodular regenerative hyperplasia of the liver: radiologic findings. J Korean Radiol Soc. 1997; 37:119–122.

5. Kim HK, Lee YH, Chung DS, Kim OD, Whang JB, Park JB. Nodular regenerative hyperplasia of the liver in an infant: case report. J Korean Radiol Soc. 2002; 47:689–692.

6. Shim SG, Sohn JH, Lee JW, et al. A case of nodular regenerative hyperplasia of liver that mimicked primary biliary cirrhosis. Korean J Hepatol. 2004; 10:313–318.
7. Wanless IR, Godwin TA, Allen F, Feder A. Nodular regenerative hyperplasia of the liver in hematologic disorders: a possible response to obliterative portal venopathy. A morphometric study of nine cases with an hypothesis on the pathogenesis. Medicine (Baltimore). 1980; 59:367–379.
8. Morris JM, Oien KA, McMahon M, et al. Nodular regenerative hyperplasia of the liver: survival and associated features in a UK case series. Eur J Gastroenterol Hepatol. 2010; 22:1001–1005.

9. Rubbia-Brandt L, Lauwers GY, Wang H, et al. Sinusoidal ob-struction syndrome and nodular regenerative hyperplasia are frequent oxaliplatin-associated liver lesions and partially pre-vented by bevacizumab in patients with hepatic colorectal metastasis. Histopathology. 2010; 56:430–439.

10. Buster EH, van Vuuren HJ, Zondervan PE, Metselaar HJ, Tilanus HW, de Man RA. Thiopurine-methyltransferase and inosine tri-phosphate pyrophosphatase polymorphism in a liver transplant recipient developing nodular regenerative hyperplasia on low-dose azathioprine. Eur J Gastroenterol Hepatol. 2008; 20:68–72.

11. Daniel F, Cadranel JF, Seksik P, et al. Azathioprine induced nodular regenerative hyperplasia in IBD patients. Gastroenterol Clin Biol. 2005; 29:600–603.

12. Ueno S, Tanabe G, Sueyoshi K, et al. Hepatic hemodynamics in a patient with nodular regenerative hyperplasia. Am J Gastroenterol. 1996; 91:1012–1015.
13. Clouet M, Boulay I, Boudiaf M, et al. Imaging features of nodular regenerative hyperplasia of the liver mimicking hepatic metastases. Abdom Imaging. 1999; 24:258–261.

14. Casillas C, Martí-Bonmatí L, Galant J. Pseudotumoral presentation of nodular regenerative hyperplasia of the liver: imaging in five patients including MR imaging. Eur Radiol. 1997; 7:654–658.

15. Reshamwala PA, Kleiner DE, Heller T. Nodular regenerative hyperplasia: not all nodules are created equal. Hepatology. 2006; 44:7–14.

16. Doğ an E, Ozgür R, Ercan V, Tekin A, Senkal O, Cevikbaş U. Nodular regenerative hyperplasia of the liver: a case report. Turk J Gastroenterol. 2003; 14:64–67.
17. Kobayashi S, Saito K, Nakanuma Y. Nodular regenerative hyperplasia of the liver in hepatocellular carcinoma. An autopsy study. J Clin Gastroenterol. 1993; 16:155–159.
Go to : 
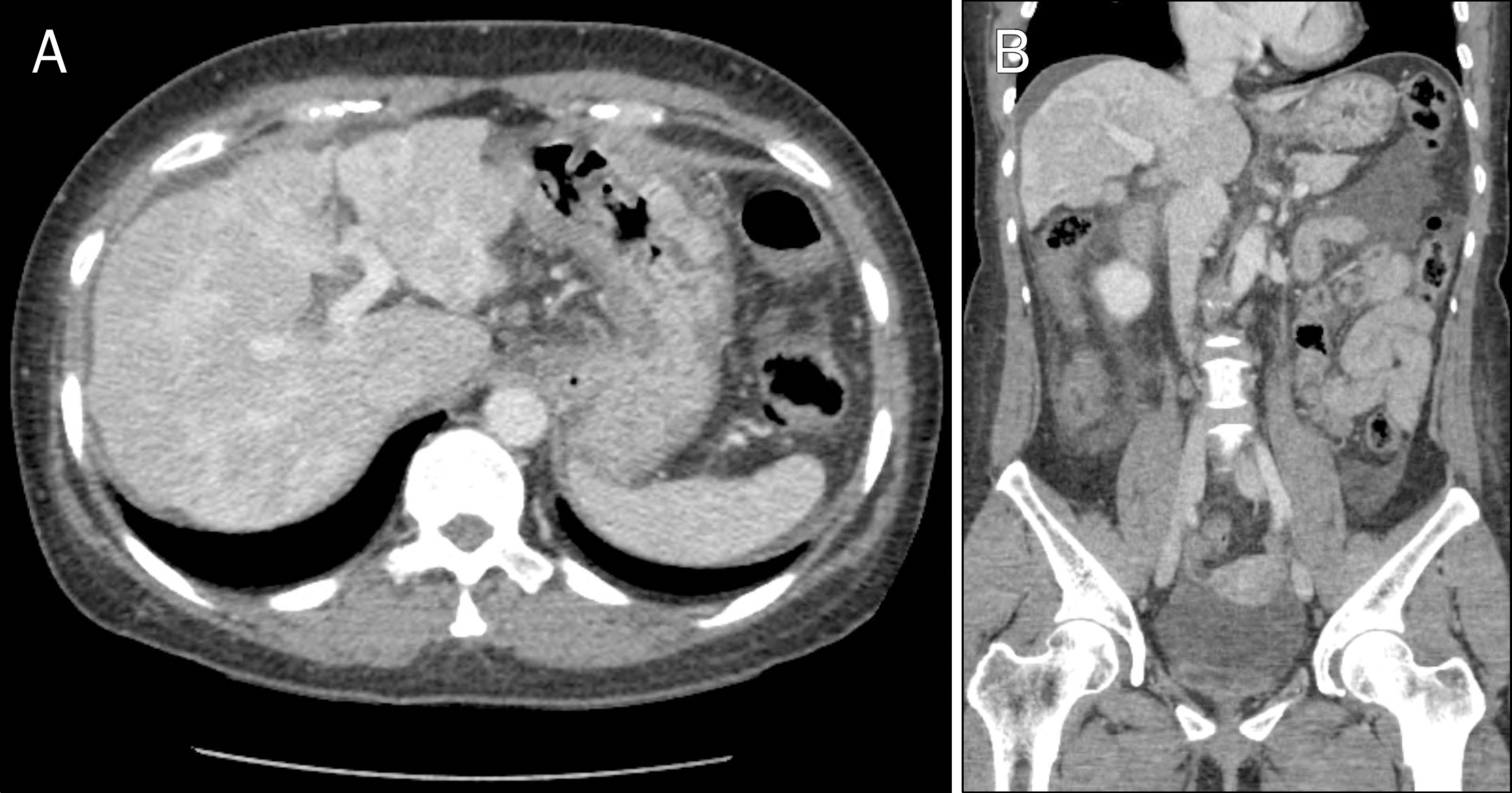 | Fig. 1.Abdominal CT shows uneven hepatic nodular lesions with decreased enhancement at portal phase (A) and moderate amount of ascites at pre-contrast phase (B). |
 | Fig. 2.Initial liver MRI shows large T2 low (A) and T1 high (B) nodular lesions in the liver. |
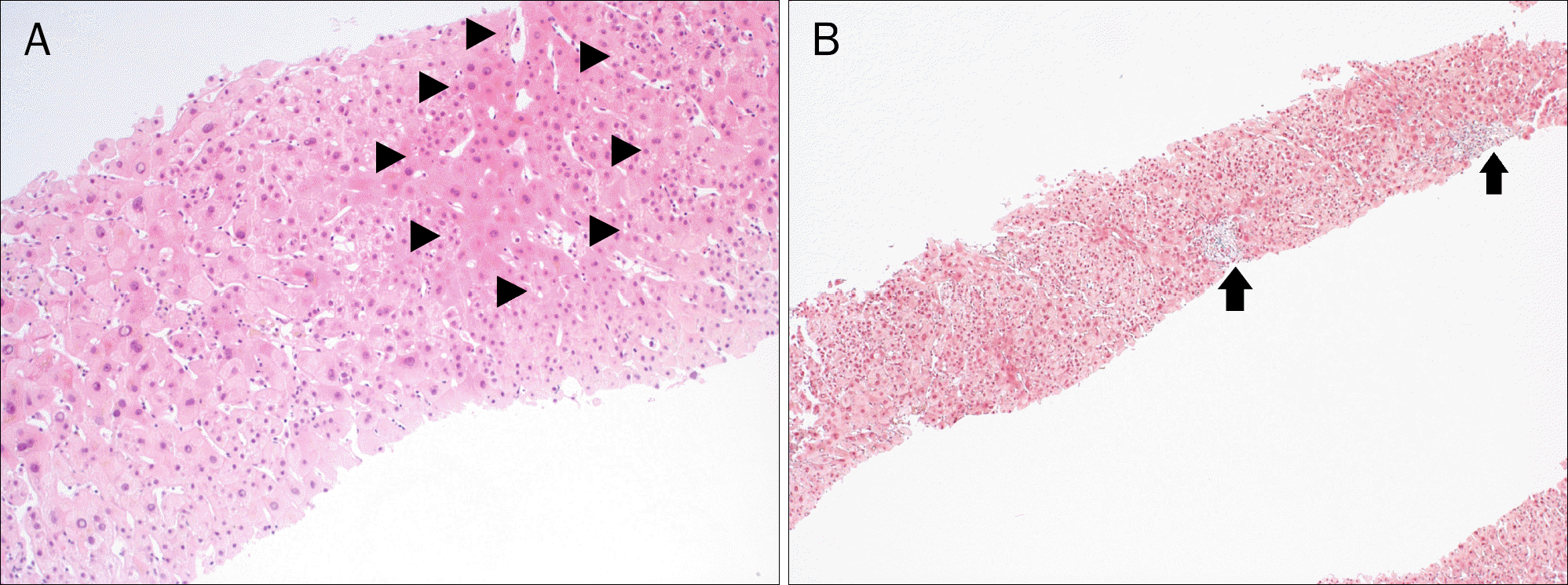 | Fig. 3.(A) Most hepatocytes are arranged in two-to-three cell thick plates, and small cell or large cell changes (arrowheads) are seen (H&E, ×100).(B) Masson trichrome stain (×40) shows absence of fibrosis and presence of portal tracts (arrows). |
 | Fig. 4.Follow-up liver MRI shows progression of nodular regenerative hyperplasia in the liver. These nodular lesions show T2 low (A) and T1 high (B) signal intensity. |
Table 1.
Roussel Uclaf Causality Assessment Method Scale Score of Our Patient




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download