Abstract
Background/Aims
Tenofovir disoproxil fumarate (TDF) plays a pivotal role in the management of drug-resistant chronic hepatitis B. However, it remains unclear whether TDF-nucleoside analogue combination therapy provides better outcomes than TDF monotherapy. This study aimed to compare the efficacy of TDF monotherapy with that of TDF-nucleoside analogue combination therapy in patients with drug-resistant chronic hepatitis B.
Methods
This retrospective cohort study included 76 patients receiving TDF-based rescue therapy for more than 12 months. Suboptimal response was defined as serum HBV-DNA level of >60 IU/mL during prior rescue therapy. Multi-drug resistance was defined as the presence of two or more drug resistance-related mutations confirmed by mutation detection assay. The relationship between baseline characteristics and virologic response (HBV DNA <20 IU/mL) at 12 months were evaluated using logistic regression analysis.
Results
Fifty-five patients (72.4%) were suboptimal responders to prior rescue therapy, and 26 (34.2%) had multi-drug resistance. Forty-two patients (55.3%) received combination therapy with nucleoside analogues. Virologic response at 12 months was not significantly different between the TDF monotherapy group and TDF-nucleoside analogue combination therapy group (p=0.098). The serum HBV DNA level was reduced to −4.49±1.67 log10 IU/mL in the TDF monotherapy group and to −3.97±1.69 log10 IU/mL in the TDF-nucleoside analogue combination therapy group at 12 months (p=0.18). In multivariate analysis, female sex (p=0.032), low baseline HBV-DNA level (p=0.013), and TDF monotherapy (p=0.046) were predictive factors for virologic response at 12 months.
References
2. Ministry of Helalth & Welfare, Korea Centers for Disease Control and Prevention. The third Korea national health and nutrition examination survey (KNHANES III), 2005: health examination. Sejong: Ministry of Helalth & Welfare;2006. p. 68.
4. Chu CM. Natural history of chronic hepatitis B virus infection in adults with emphasis on the occurrence of cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol. 2000; 15(Suppl):E25–E30.

5. Dienstag JL, Schiff ER, Wright TL, et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999; 341:1256–1263.

6. Liaw YF, Sung JJ, Chow WC, et al. Cirrhosis Asian Lamivudine Multicentre Study Group. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004; 351:1521–1531.

7. Lai CL, Dienstag J, Schiff E, et al. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin Infect Dis. 2003; 36:687–696.

8. Liaw YF, Gane E, Leung N, et al. GLOBE Study Group. 2-Year GLOBE trial results: telbivudine Is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009; 136:486–495.

9. Gallego A, Sheldon J, García-Samaniego J, et al. Evaluation of initial virological response to adefovir and development of adefovir-resistant mutations in patients with chronic hepatitis B. J Viral Hepat. 2008; 15:392–398.

10. Lee YS, Suh DJ, Lim YS, et al. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology. 2006; 43:1385–1391.

11. Yim HJ, Seo YS, Yoon EL, et al. Adding adefovir vs. switching to entecavir for lamivudine-resistant chronic hepatitis B (ACE study): a 2-year follow-up randomized controlled trial. Liver Int. 2013; 33:244–254.

12. Lim YS, Lee JY, Lee D, et al. Randomized trial of the virologic response during up to two years of entecavir-adefovir combination therapy in multiple-drug-refractory chronic hepatitis B virus patients. Antimicrob Agents Chemother. 2013; 57:3369–3374.

13. Gordon SC, Krastev Z, Horban A, et al. Efficacy of tenofovir disoproxil fumarate at 240 weeks in patients with chronic hepatitis B with high baseline viral load. Hepatology. 2013; 58:505–513.

14. Kitrinos KM, Corsa A, Liu Y, et al. No detectable resistance to tenofovir disoproxil fumarate after 6 years of therapy in patients with chronic hepatitis B. Hepatology. 2014; 59:434–442.

15. Fung S, Kwan P, Fabri M, et al. Randomized comparison of tenofovir disoproxil fumarate vs emtricitabine and tenofovir disoproxil fumarate in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology. 2014; 146:980–988.e1.

16. Seto WK, Liu K, Wong DK, et al. Patterns of hepatitis B surface antigen decline and HBV DNA suppression in Asian treatment-experienced chronic hepatitis B patients after three years of tenofovir treatment. J Hepatol. 2013; 59:709–716.

17. Korean Association for the Study of the Liver. KASL clinical practice guidelines: management of chronic hepatitis B. Clin Mol Hepatol. 2012; 18:109–162.
18. Shim JH, Suh DJ, Kim KM, et al. Efficacy of entecavir in patients with chronic hepatitis B resistant to both lamivudine and adefovir or to lamivudine alone. Hepatology. 2009; 50:1064–1071.

19. Cho SW, Koh KH, Cheong JY, et al. Low efficacy of entecavir therapy in adefovir-refractory hepatitis B patients with prior lamivudine resistance. J Viral Hepat. 2010; 17:171–177.

20. Kim MC, Jung SW, Shin JW, Park NH. The level of HBV DNA at month 12 is an important predictor of virological breakthrough during adefovir monotherapy in chronic hepatitis B patients with lamivudine resistance. Dig Dis Sci. 2011; 56:1215–1221.

21. Patterson SJ, George J, Strasser SI, et al. Tenofovir disoproxil fumarate rescue therapy following failure of both lamivudine and adefovir dipivoxil in chronic hepatitis B. Gut. 2011; 60:247–254.

22. Berg T, Zoulim F, Moeller B, et al. Long-term efficacy and safety of emtricitabine plus tenofovir DF vs. tenofovir DF monotherapy in adefovir-experienced chronic hepatitis B patients. J Hepatol. 2014; 60:715–722.

23. Heathcote EJ, Marcellin P, Buti M, et al. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology. 2011; 140:132–143.

24. Yeon JE, Yoo W, Hong SP, et al. Resistance to adefovir dipivoxil in lamivudine resistant chronic hepatitis B patients treated with adefovir dipivoxil. Gut. 2006; 55:1488–1495.

25. Ijaz S, Arnold C, Dervisevic S, et al. Dynamics of lamivudine-re-sistant hepatitis B virus during adefovir monotherapy versus lamivudine plus adefovir combination therapy. J Med Virol. 2008; 80:1160–1170.

26. Zoulim F. Mechanism of viral persistence and resistance to nucleoside and nucleotide analogs in chronic hepatitis B virus infection. Antiviral Res. 2004; 64:1–15.

27. van Bömmel F, de Man RA, Wedemeyer H, et al. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-mono-infected patients after failure of nucleoside/nucleotide analogues. Hepatology. 2010; 51:73–80.

28. Petersen J, Ratziu V, Buti M, et al. Entecavir plus tenofovir combination as rescue therapy in pre-treated chronic hepatitis B patients: an international multicenter cohort study. J Hepatol. 2012; 56:520–526.

29. Keeffe EB, Zeuzem S, Koff RS, et al. Report of an international workshop: roadmap for management of patients receiving oral therapy for chronic hepatitis B. Clin Gastroenterol Hepatol. 2007; 5:890–897.

30. Shin JW, Jung SW, Park BR, et al. HBV DNA level at 24 weeks is the best predictor of virological response to adefovir add-on therapy in patients with lamivudine resistance. Antivir Ther. 2012; 17:387–394.

31. Lee JM, Park JY, Kim do Y, et al. Long-term adefovir dipivoxil monotherapy for up to 5 years in lamivudine-resistant chronic hepatitis B. Antivir Ther. 2010; 15:235–241.

32. Yang YJ, Shim JH, Kim KM, Lim YS, Lee HC. Assessment of current criteria for primary nonresponse in chronic hepatitis B patients receiving entecavir therapy. Hepatology. 2014; 59:1303–1310.

33. European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B. J Hepatol. 2009; 50:227–242.
Fig. 1.
Mean (95% CI) changes in serum HBV DNA levels between TDF monotherapy group and TDF-nucleoside analogue combination therapy group.
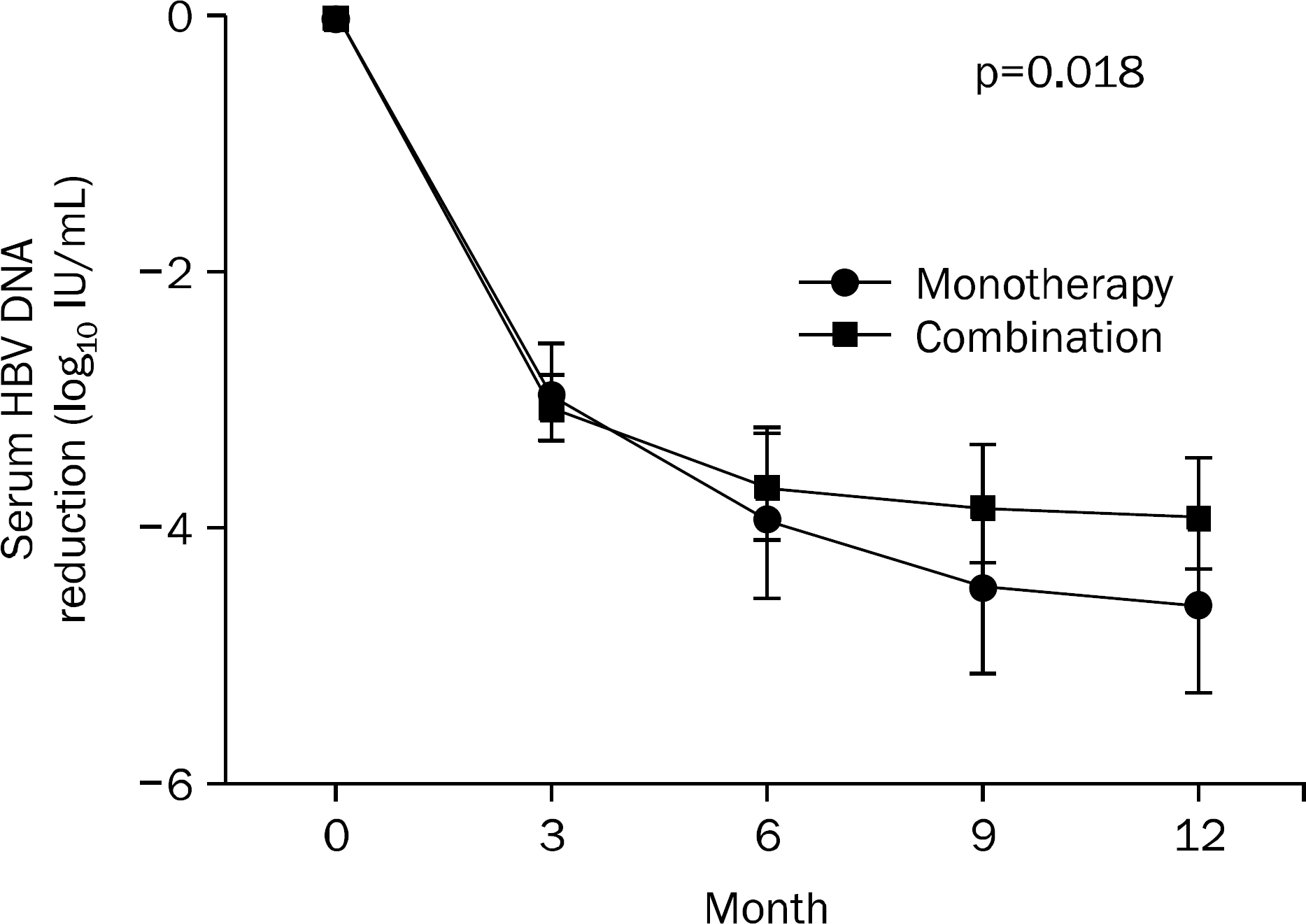
Fig. 2.
Mean (95% CI) changes in serum HBV DNA levels according to adefovir dipivoxil resistance-associated mutations. ADV-R, adefovir dipivoxil resistance-associate mutation.
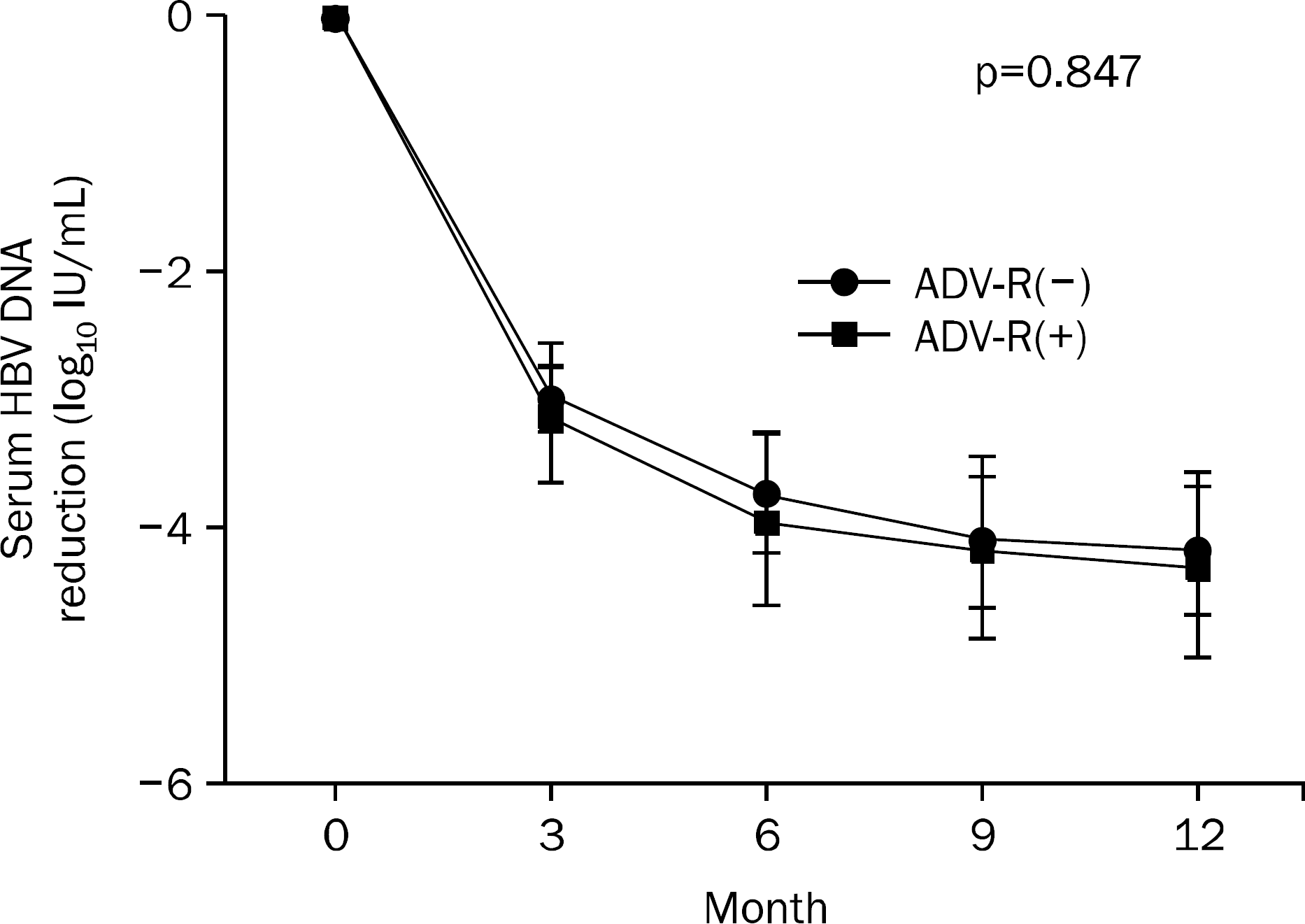
Table 1.
Baseline Characteristics of the Study Population
Table 2.
Baseline Mutation Patterns and Virologic Response
Table 3.
Predictive Factors for Virological Response at 12 Months




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download