Abstract
Background/Aims
Performance of polyethylene glycol solution (PEG) is often unsatisfactory as bowel preparation agent for colonoscopy. In order to provide equivalent efficacy with better patient tolerance, sodium phosphate tablet (SPT) has been developed. This study was carried out to compare the efficacy and compliance of two bowel preparation methods: PEG with ascorbic acid (PEGA) vs. SPT preparation.
Methods
A multicenter, randomized controlled trial was performed. Primary efficacy variable was overall quality of colon cleansing assessed by Boston bowel preparation scale (BBPS) during colonoscopy. Patient's satisfaction and adverse events were evaluated by means of symptom questionnaire completed by each patient immediately before colonoscopy.
Results
A total of 189 patients were randomly assigned to undergo pre-colonoscopic bowel preparation with either SPT (n=96) or PEGA (n=93). Overall BBPS score was 8.3±1.12 in the SPT group and 8.4±0.96 in the PEGA group (p=0.441). Among the 189 patients, 90 had polyps (47.6%) and 50 had adenomas (26.5%). The polyp/adenoma detection rate was 54.2% (n=52)/27.1% (n=26) for SPT group and 40.9% (n=38)/25.8% (n=24) for PEGA group (p=0.079 and 0.790, respectively). More number of patients were unable to take the prescribed dose of PEGA compared with the SPT regimen (8.6% vs. 2.0%, p=0.045). Overall satisfaction score was 7.9±1.63 in the SPT group and 7.4±1.53 in the PEGA group (p=0.022).
Go to : 
References
1. Shin A, Kim KZ, Jung KW, et al. Increasing trend of colorectal cancer incidence in Korea, 1999–2009. Cancer Res Treat. 2012; 44:219–226.

2. Yoo JR, Song HJ, Beom JW, et al. Predictable factors of early colorectal cancer after colonoscopic polypectomy. Intest Res. 2013; 11:169–177.

3. Kim SE, Hong SP, Kim HS, et al. Multi-Society Task Force for Development of Guidelines for Colorectal Polyp Screening, Surveillance and Management. A Korean national survey for colorectal cancer screening and polyp diagnosis methods using web-based survey. Korean J Gastroenterol. 2012; 60:26–35.

4. Chen TA, Wong HY, Lin CK, et al. High-dose bisacodyl plus water lavage compared with oral sodium phosphate as bowel preparation for outpatient colonoscopy. J Chin Med Assoc. 2009; 72:402–407.

5. Kim CJ, Jung YS, Park JH, et al. Prevalence, clinicopathologic characteristics, and predictors of interval colorectal cancers in Korean population. Intest Res. 2013; 11:178–183.

6. Cha JM. Colonoscopy quality is the answer for the emerging is-sue of interval cancer. Intest Res. 2014; 12:110–116.

7. Davis GR, Santa Ana CA, Morawski SG, Fordtran JS. Development of a lavage solution associated with minimal water and electrolyte absorption or secretion. Gastroenterology. 1980; 78:991–995.

8. Choi NK, Lee J, Chang Y, et al. Polyethylene glycol bowel preparation does not eliminate the risk of acute renal failure: a pop-ulation-based case-crossover study. Endoscopy. 2013; 45:208–213.

9. Yoon JH, Park DI, Shin JE, et al. Comparison of bowel preparation depending on completion time of polyethylene glycol ingestion and start time of colonoscopy. Intest Res. 2010; 8:24–29.

10. DiPalma JA, Brady CE 3rd. Colon cleansing for diagnostic and surgical procedures: polyethylene glycol-electrolyte lavage solution. Am J Gastroenterol. 1989; 84:1008–1016.
12. Park JB, Lee YK, Yang CH. The evolution of bowel preparation and new developments. Korean J Gastroenterol. 2014; 63:268–275.

13. Park S, Lim YJ. Adjuncts to colonic cleansing before colonoscopy. World J Gastroenterol. 2014; 20:2735–2740.

14. Balaban DH, Leavell BS Jr, Oblinger MJ, Thompson WO, Bolton ND, Pambianco DJ. Low volume bowel preparation for colonoscopy: randomized, endoscopist-blinded trial of liquid sodium phosphate versus tablet sodium phosphate. Am J Gastroenterol. 2003; 98:827–832.

15. Aihara H, Saito S, Arakawa H, et al. Comparison of two sodium phosphate tablet-based regimens and a polyethylene glycol regimen for colon cleansing prior to colonoscopy: a randomized prospective pilot study. Int J Colorectal Dis. 2009; 24:1023–1030.

16. Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009; 69:620–625.

17. Juluri R, Eckert G, Imperiale TF. Meta-analysis: randomized controlled trials of 4-L polyethylene glycol and sodium phosphate solution as bowel preparation for colonoscopy. Aliment Pharmacol Ther. 2010; 32:171–181.

18. Gurudu SR, Li F, Fleischer DE, et al. Patient preference and acceptance with sodium phosphate tablet preparation for colonoscopy. Dig Dis Sci. 2009; 54:1555–1559.

19. Kastenberg D, Barish C, Burack H, et al. INKP-100 Study Group. Tolerability and patient acceptance of sodium phosphate tablets compared with 4-L PEG solution in colon cleansing: combined results of 2 identically designed, randomized, controlled, parallel group, multicenter phase 3 trials. J Clin Gastroenterol. 2007; 41:54–61.
20. Kim EJ, Park YI, Kim YS, et al. A Korean experience of the use of Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Saudi J Gastroenterol. 2014; 20:219–224.

21. Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. 2003; 58:76–79.

22. Lebwohl B, Kastrinos F, Glick M, Rosenbaum AJ, Wang T, Neugut AI. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointest Endosc. 2011; 73:1207–1214.

23. Chokshi RV, Hovis CE, Hollander T, Early DS, Wang JS. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc. 2012; 75:1197–1203.

Go to : 
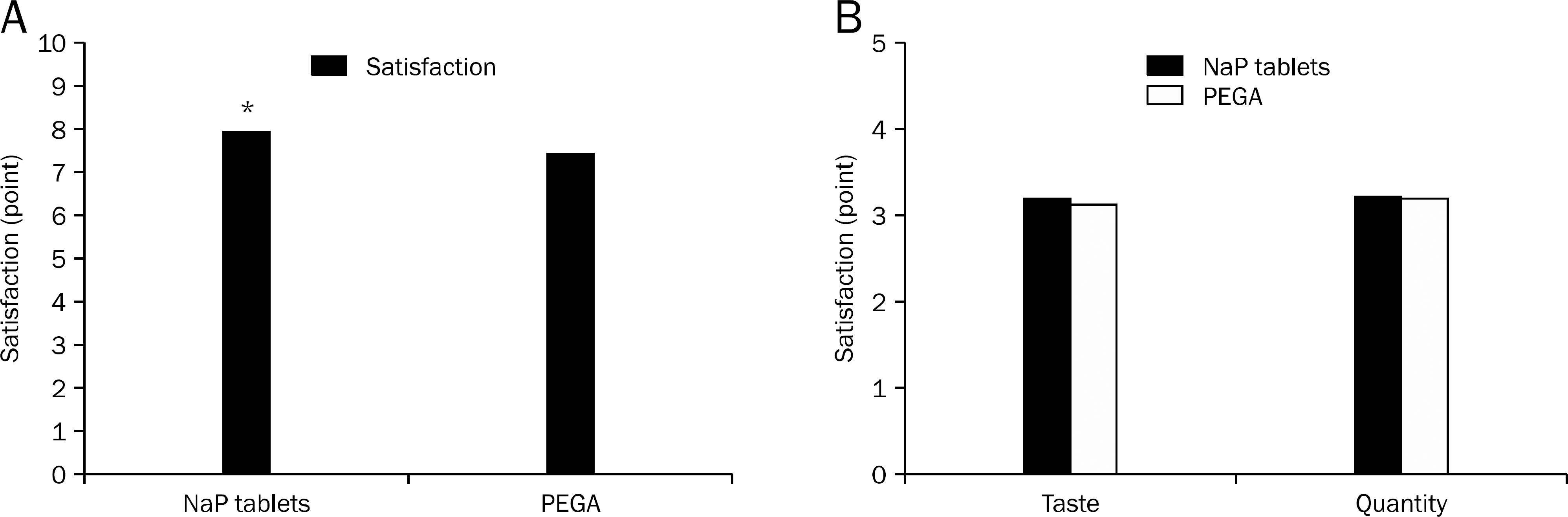 | Fig. 1.Comparison of patient's satisfaction. (A) Patients preferred NaP tablet rather than PEGA. (B) There is no significant difference between groups for taste and quantity (p=0.864, p=0.488). ∗p=0.022. NaP, sodium phosphate; PEGA, polyethylene glycol with ascorbic acid. |
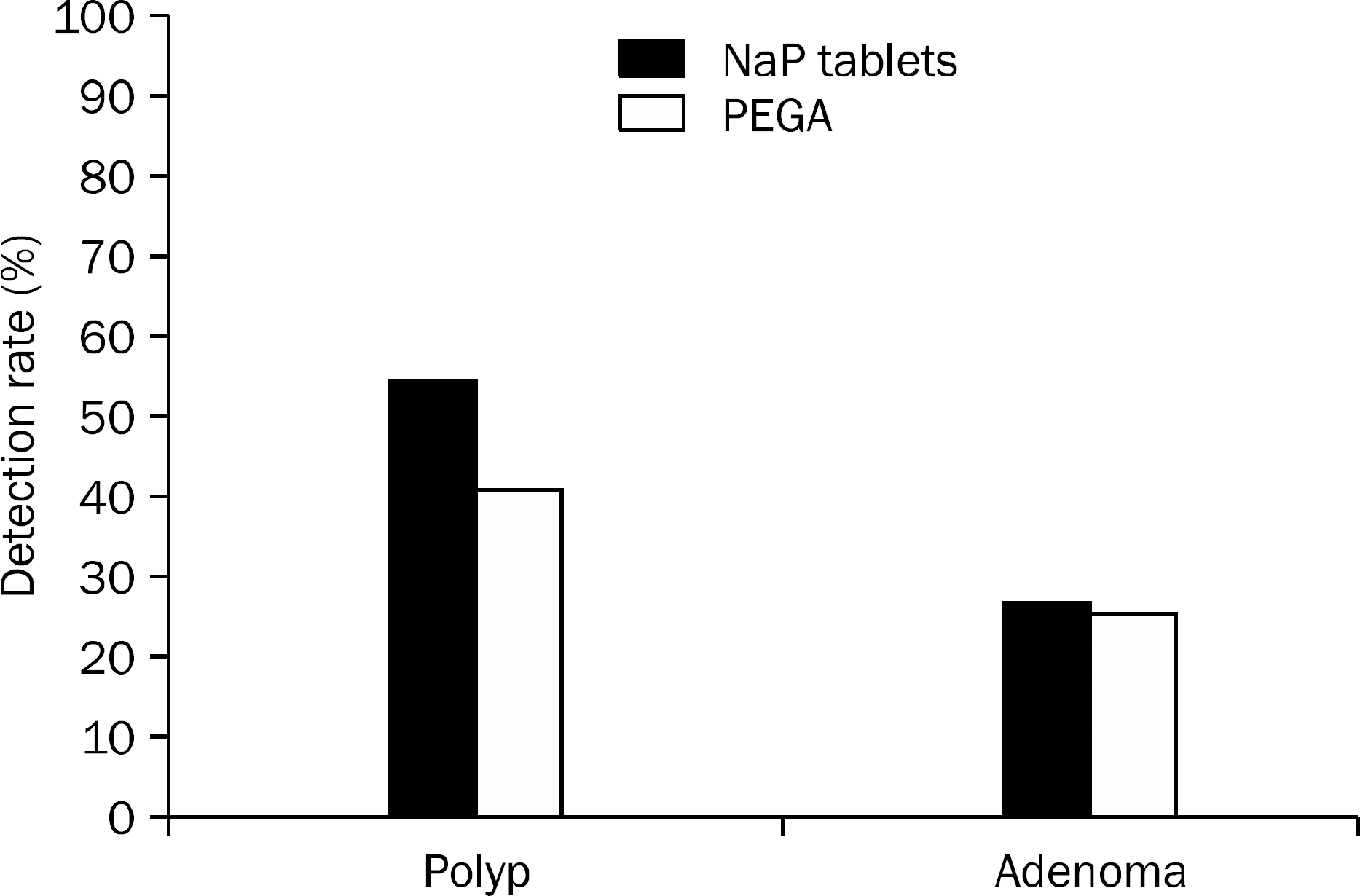 | Fig. 2.Detection rate of polyp and adenoma between the two groups. There is no significant difference between two groups in polyp detection rate (p=0.079) and adenoma detection rate (p=0.790). NaP, sodium phosphate; PEGA, polyethylene glycol with ascorbic acid. |
Table 1.
Baseline Characteristics
Table 2.
Procedural Time and Bowel Preparation




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download