Abstract
A pyogenic pancreatic abscess mimicking pancreatic neoplasm in the absence of acute pancreatitis is rare. We report four patients who each presented with a pancreatic mass at the pancreas head or body without acute pancreatitis. The presenting symptoms were abdominal pain, fever, or weight loss. Abdominal CT scans showed low-density round masses at the pancreas head or body with/without lymphadenopathy. In each case, a PET-CT scan showed a mass with a high SUV, indicating possible malignancy. Comorbid diseases were identified in all patients: chronic pancreatitis and thrombus at the portal vein, penetrating duodenal ulcer, distal common bile duct stenosis, and diabetes mellitus. Diagnoses were performed by laparoscopic biopsy in two patients and via EUS fine needle aspiration in one patient. One patient revealed a multifocal microabscess at the pancreatic head caused by a deep-penetrating duodenal ulcer. He was treated with antibiotics and a proton-pump inhibitor. The clinical symptoms and pancreatic images of all the patients were improved using conservative management. Infective causes should be considered for a pancreatic mass mimicking malignancy.
References
1. Goh BK, Jeyaraj PR, Chan HS, et al. A case of fish bone perforation of the stomach mimicking a locally advanced pancreatic carcinoma. Dig Dis Sci. 2004; 49:1935–1937.
2. An CH, Kim KH, Kim JS, Kim JI. Pancreatic abscess caused by gastric perforation. ANZ J Surg. 2007; 77:709.

4. Chong VH. Isolated pyogenic pancreatic abscess mimicking a neoplasm. JOP. 2008; 9:309–312.
5. Safioleas M, Stamatakos MK, Mouzopoulos GJ, et al. Pancreatic abscess due to perforation of duodenal diverticulum. Chirurgia (Bucur). 2006; 101:523–524.
6. Hu J, Sheu MH, Yang WC, Li JC, Ng YY. Peritonitis and pancreatic abscess in a CAPD patient. Perit Dial Int. 2002; 22:430–431.

7. Seo KS, Oh HM, Hong JH, et al. A case of pancreatic abscess due to Salmonella typhi. Korean J Med. 1998; 54:101–104.
9. Garg P, Parashar S. Pancreatic abscess due to Salmonella typhi. Postgrad Med J. 1992; 68:294–295.

10. Taguchi M, Nishikawa S, Matsuoka H, et al. Pancreatic abscess caused by Corynebacterium coyleae mimicking malignant neoplasm. Pancreas. 2006; 33:425–429.

11. Eliashiv A, Olumide F, Norton L, Eiseman B. Depression of cell-mediated immunity in diabetes. Arch Surg. 1978; 113:1180–1183.

Fig. 1.
Image studies of Case 1. (A) Axial view of the abdominal CT scan. A 2.7-cm ill-defined low-density mass (arrow) with peripancreatic fat infiltration was located at the pancreatic body. (B) Coronal view of the abdominal CT scan. An ill-defined, soft tissue density lesion around the celiac axis, common hepatic artery, splenic artery, and portal vein was noted (arrow). (C) PET-CT scan. Hot uptake (SUV 3.8) at the pancreatic body, indicating possible malignancy, was observed (arrow).
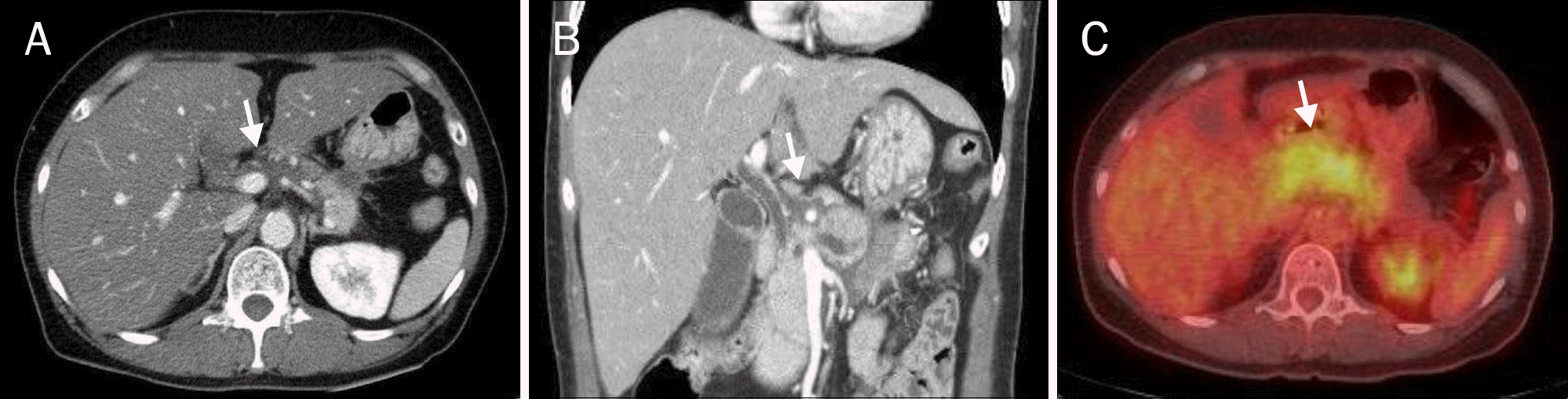
Fig. 2.
Image studies of Case 2. (A) Axial view of the abdominal CT scan. A 5.0-cm well-defined low-density mass with heterogenous enhancement at the pancreas head (arrow), parenchymal atrophy, and a calcifying stone in the dilated pancreatic duct (arrowhead). (B) Coronal view of the abdominal CT scan. The portal vein and common hepatic artery were encased by a low-density mass (arrow). (C) Axial view of the abdominal CT scan after treatment. Resolution of the abscess was noted (arrowhead).
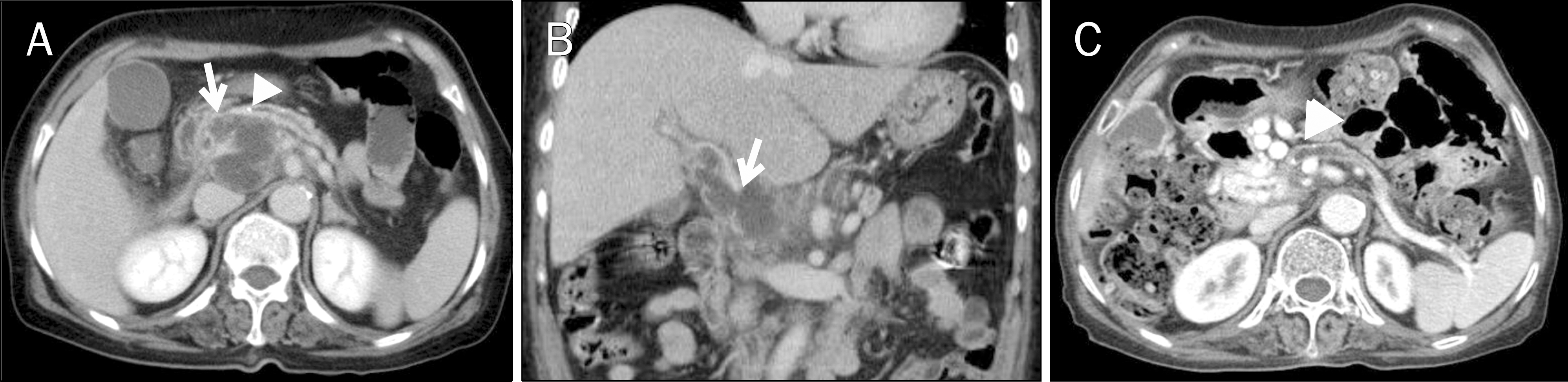
Fig. 3.
Image studies of Case 3. (A, B) Axial and coronal views of the abdominal CT scan. A 1.2-cm well-defined low-density mass at the pancreatic head was observed (arrows). (C) Endoscopic ultrasonography. A 1.2-cm-sized anechoic round mass was seen at the pancreas head (arrows).

Fig. 4.
Image studies of Case 4. (A) Axial view of the abdominal CT scan. A 1.7-cm multifocal ill-defined low-density lesion with multiple enhancing tubular structures at the head of the pancreas was observed (arrow). (B) PET-CT scan. Hot uptake (SUVmax 3.8) at the pancreatic head indicated possible malignancy or inflammation (arrow). (C, D) Endoscopic ultrasonography. A 2.8×1.6 cm-sized round isoechoic lesion with internal anechoic tubular structures was observed using vascular doppler (arrows). (E) Endoscopic finding. A 3.0-cm round deep-penetrating duodenal ulcer with a central pit was observed (arrow). (F) Endoscopic finding one year later. The duodenal ulcer had improved; however, the duodenal fistula remained (arrow).
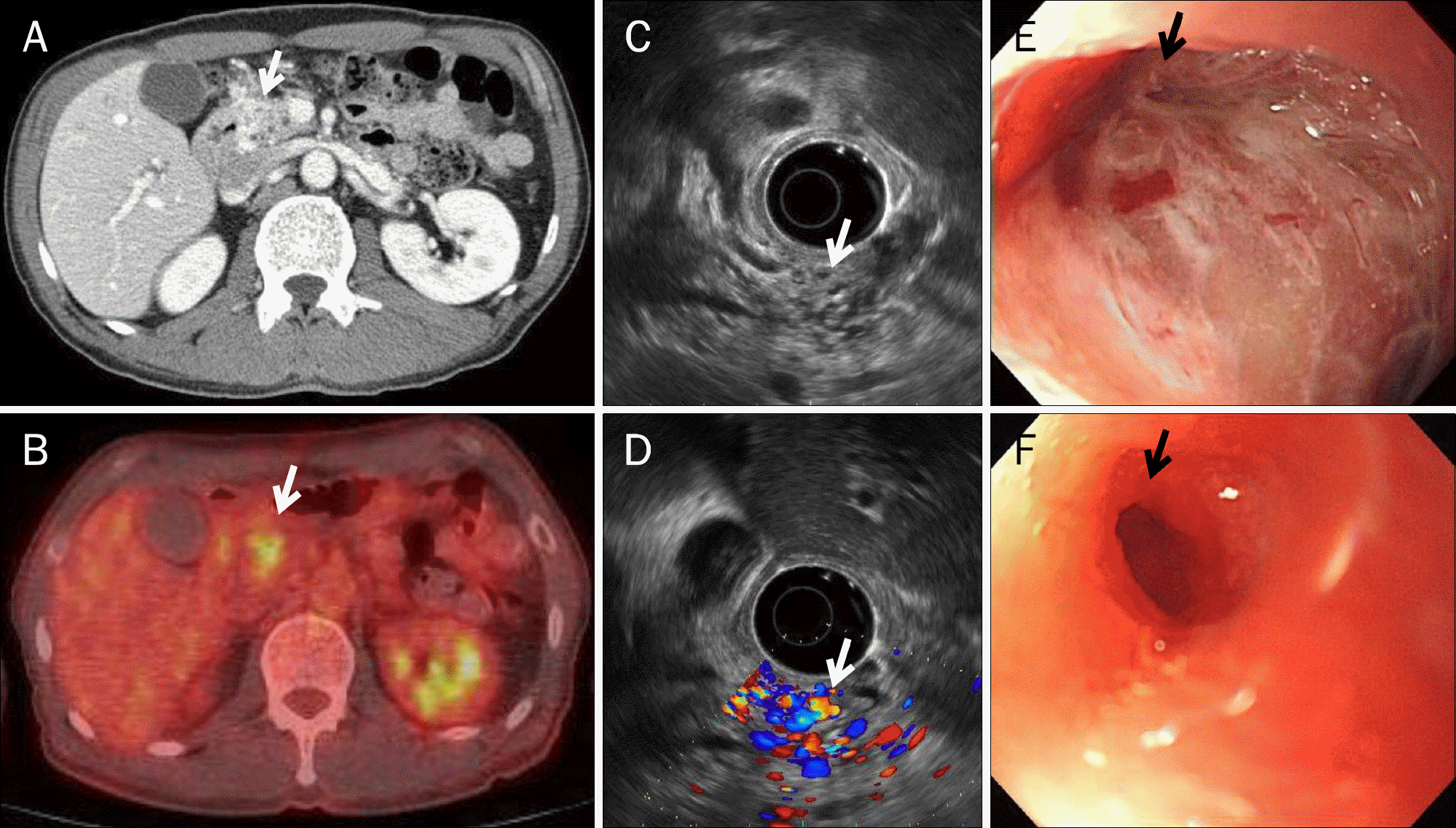
Table 1.
Baseline and Clinical Characteristics of the Four Patients




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download