Abstract
With an increasing number of patients with hepatocellular carcinoma (HCC) undergoing liver transplantation (LT), tumor recurrence remains the main limiting factor for long-term survival. Although sorafenib is available for advanced HCC, there is still a lack of data on the use of sorafenib for treatment of recurrent HCC after LT. Here, we report on four cases of the use of sorafenib for treatment of recurrent HCC after LT. The median time of recurrence from LT was 4 months (range, 1–16 months). Two of the four evaluated patients showed stable disease, which was the best response and the duration of stabilization was 11 months and 5 months, respectively. One patient also experienced stable disease and remained in stable disease without sorafenib therapy for 29 months and the total duration of stabilization was 38 months. The remaining patient showed partial response but stopped treatment due to radiological tumor progression during treatment. Although all cases were high risk group for recurrence such as above Milan criteria, vascular invasion and tumor biology, clinical outcomes showed some good results. Therefore, sorafenib may be an acceptable treatment option for recurrent HCC after LT.
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.

2. Llovet JM, Fuster J, Bruix J. Barcelona-Clínic Liver Cancer Group. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004; 10(2 Suppl 1):S115–S120.

3. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009; 10:25–34.

5. Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996; 334:693–699.

6. Mazzaferro V, Llovet JM, Miceli R, et al. Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009; 10:35–43.
7. Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001; 33:1394–1403.

8. DuBay D, Sandroussi C, Sandhu L, et al. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg. 2011; 253:166–172.

9. Guiteau JJ, Cotton RT, Washburn WK, et al. An early regional experience with expansion of Milan Criteria for liver transplant recipients. Am J Transplant. 2010; 10:2092–2098.

10. Duffy JP, Vardanian A, Benjamin E, et al. Liver transplantation criteria for hepatocellular carcinoma should be expanded: a 22-year experience with 467 patients at UCLA. Ann Surg. 2007; 246:502–509. discussion 509–511.
11. Wilhelm S, Carter C, Lynch M, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006; 5:835–844.

12. Llovet JM, Ricci S, Mazzaferro V, et al. SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390.

13. Bhoori S, Toffanin S, Sposito C, et al. Personalized molecular tar-geted therapy in advanced, recurrent hepatocellular carcinoma after liver transplantation: a proof of principle. J Hepatol. 2010; 52:771–775.

14. Lee JO, Kim DY, Lim JH, et al. Palliative chemotherapy for patients with recurrent hepatocellular carcinoma after liver transplantation. J Gastroenterol Hepatol. 2009; 24:800–805.

15. Wang Y, Speeg KV, Washburn WK, Halff G. Sirolimus plus sorafenib in treating HCC recurrence after liver transplantation: a case report. World J Gastroenterol. 2010; 16:5518–5522.

16. Yoon DH, Ryoo BY, Ryu MH, et al. Sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Jpn J Clin Oncol. 2010; 40:768–773.

17. Waidmann O, Hofmann WP, Zeuzem S, Trojan J. mTOR inhibitors and sorafenib for recurrent heptocellular carcinoma after ortho-topic liver transplantation. J Hepatol. 2011; 54:396–398.

18. Sposito C, Mariani L, Germini A, et al. Comparative efficacy of sorafenib versus best supportive care in recurrent hepatocellular carcinoma after liver transplantation: a case-control study. J Hepatol. 2013; 59:59–66.

Fig. 1.
(A-D) The radiologic features of Case 1. (A, B) Chest CT showed multiple lung nodules on both lobes (arrows) before sorafenib treatment.(C, D) Two months after sorafenib administration, chest CT showed that the majority of variable sized multiple nodules on both lungs (arrows) had disappeared and some indeterminate nodules remained. (E-H) The radiologic features of Case 2. Chest CT (E, F) showed a 19 mm sized dense round nodule (arrows) in the right lower lobe (RLL) and a 3 mm sized indeterminate nodule in the left upper lobe (LUL). (G) PET-CT also showed a hypermetabolic nodule (SUVmax 3.4) on RLL (arrow). (H) Two months after sorafenib administration, chest CT showed no interval change of lung nodule in LUL (arrow).

Fig. 2.
(A-D) The radiologic features of Case 3. (A, B) Chest CT showed variable sized multiple nodules on both lungs (largest, 12 mm) (arrows) before sorafenib treatment. (C, D) Five months after sorafenib administration, chest CT revealed that several variable sized multiple nodules on both lungs had decreased and some nodules remained (arrows). (E-H) The radiologic features of Case 4. (E) Abdomen CT showed an enhancing mass (4.0×2.9 cm) at the right acetabulum. (F, G) Bone scan and PET-CT also showed bone metastasis in the right pelvic bone. (H) Three month after sorafenib administration, abdomen CT showed interval decrease in the size of metastasis at the right acetabulum.
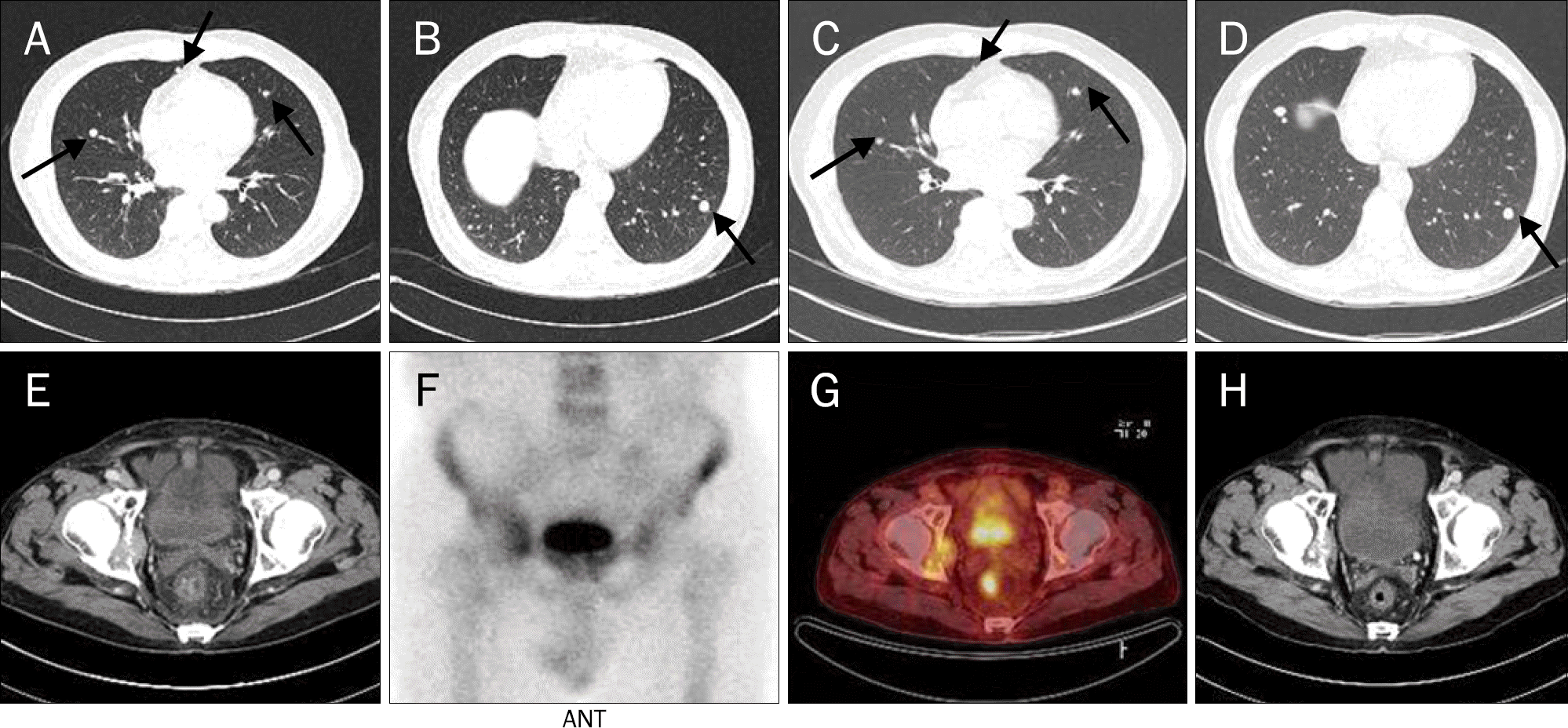
Table 1.
Demographics and Clinical Characteristics of the Patients
Table 2.
Characteristics of Patients Treated with Sorafenib for Recurrent Hepatocellular Carcinoma after Liver Transplantation




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download