Abstract
Background/Aims
Visceral larva migrans, caused by Toxocara canis and Toxocara cati, has emerged as a significant cause of eosinophilic liver abscess (ELA). Differentiation of ELA associated with toxocariasis (ELA-T) from metastasis or primary liver malignancy is sometimes difficult. However, the role of albendazole treatment remains uncertain in this condition. The aim of this study was to evaluate whether albendazole can enhance the radiologic resolution of ELA-T.
Methods
We retrospectively reviewed the medical records of the patients diagnosed with ELA-T at our institution between January 2008 and December 2011. ELA-T was diagnosed based on the imaging findings on computed tomography or magnetic resonance imaging and the presence of positive serum IgG antibody for Toxocara canis. Among a total of 163 patients, 32 patients received albendazole (albendazole group) and 131 did not (control group). Baseline characteristics and fate of liver nodules were compared between the two groups.
Results
Baseline characteristics (age, sex, number and maximal size of lesions, eosinophil count) were similar between the two groups. Median duration for achieving radiologic resolution in the albendazole group was significantly shorter than in the control group (207 days [range 186–228] vs. 302 days [range 224–380], p=0.023). In Cox regression analysis of the cumulative rates of radiologic resolution, the hazard ratio for albendazole treatment was 1.99 (95% confidence interval, 1.22-3.23).
Go to : 
References
1. Jang JK, Paik SW, Choi MS, et al. Clinical characteristics of 19 cases of biopsy-proven eosinophilic liver abscess: single center experience. Korean J Gastroenterol. 2001; 38:37–41.
2. Jang HJ, Lee WJ, Lee SJ, Kim SH, Lim HK, Lim JH. Focal eosinophilic necrosis of the liver in patients with underlying gastric or colorectal cancer: CT differentiation from metastasis. Korean J Radiol. 2002; 3:240–244.

3. Yoo SY, Han JK, Kim YH, Kim TK, Choi BI, Han MC. Focal eosinophilic infiltration in the liver: radiologic findings and clinical course. Abdom Imaging. 2003; 28:326–332.

4. Kim YK, Kim CS, Moon WS, Cho BH, Lee SY, Lee JM. MRI findings of focal eosinophilic liver diseases. Am J Roentgenol. 2005; 184:1541–1548.

5. Chang S, Lim JH, Choi D, et al. Hepatic visceral larva migrans of Toxocara canis: CT and sonographic findings. Am J Roentgenol. 2006; 187:W622–W629.
7. Kim WH, Kim SH, Kim YH, et al. Fluorine-18-FDG PET findings of focal eosinophilic liver disease: correlation with CT and/or MRI, laboratory, and pathologic findings. Abdom Imaging. 2010; 35:437–446.

9. Hartleb M, Januszewski K. Severe hepatic involvement in visceral larva migrans. Eur J Gastroenterol Hepatol. 2001; 13:1245–1249.

10. Leone N, Baronio M, Todros L, et al. Hepatic involvement in larva migrans of Toxocara canis: report of a case with pathological and radiological findings. Dig Liver Dis. 2006; 38:511–514.

11. Hossack J, Ricketts P, Te HS, Hart J. A case of adult hepatic toxocariasis. Nat Clin Pract Gastroenterol Hepatol. 2008; 5:344–348.

12. Kaplan KJ, Goodman ZD, Ishak KG. Eosinophilic granuloma of the liver: a characteristic lesion with relationship to visceral larva migrans. Am J Surg Pathol. 2001; 25:1316–1321.
13. Pawlowski Z. Toxocariasis in humans: clinical expression and treatment dilemma. J Helminthol. 2001; 75:299–305.

14. Kim YS, Park SJ, Kim HK, Park JM. A case of eosinophilic abscess mistaken for metastasis due to FDG uptake in PET-CT. Korean J Gastroenterol. 2009; 54:349–354.

15. Won JH, Kim MJ, Kim BM, et al. Focal eosinophilic infiltration of the liver: a mimick of hepatic metastasis. Abdom Imaging. 1999; 24:369–372.

16. Park S, Kim YS, Kim YJ, et al. Toxocariasis masquerading as liver and lung metastatic nodules in patents with gastrointestinal cancer: clinicopathologic study of five cases. Dig Dis Sci. 2012; 57:155–160.

17. Jin SY. Eosinophilic Liver Abscess. Korean J Hepatol. 2005; 11:176–179.
18. Shin YM. Hepatic eosinophilic abscess presenting as a single nodular mass. Korean J Hepatol. 2010; 16:95–99.

19. Jung JK, Jung JT, Lee CH, Kim EY, Kwon JG, Kim BS. A case of hepatic abscess caused by toxocara. Korean J Hepatol. 2007; 13:409–413.

20. Despommier D. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev. 2003; 16:265–272.

21. Kwon NH, Oh MJ, Lee SP, Lee BJ, Choi DC. The prevalence and diagnostic value of toxocariasis in unknown eosinophilia. Ann Hematol. 2006; 85:233–238.

22. Magnaval JF, Glickman LT, Dorchies P, Morassin B. Highlights of human toxocariasis. Korean J Parasitol. 2001; 39:1–11.

Go to : 
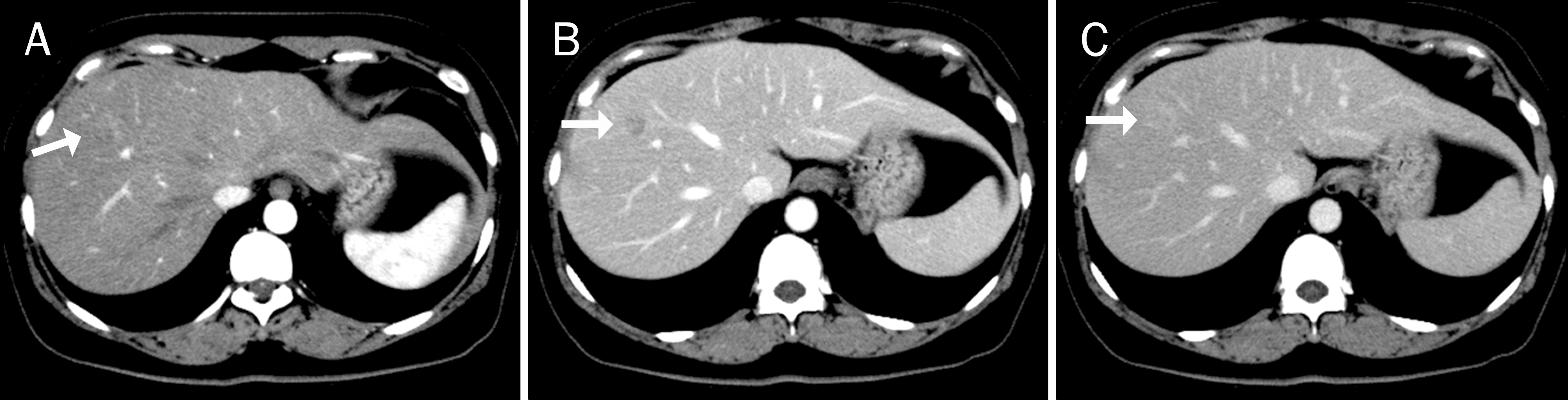 | Fig. 1.CT findings in a 51-year-old female patient with an eosinophilic liver abscess. (A) A 1.4-cm nodule (arrow) was poorly delineated in the arterial phase. (B) It presented as an ill-defined low-attenuation nodule (arrow) in the portal phase. (C) It was also invisible (arrow) in the equilibrium phase. |
 | Fig. 2.Image findings in a 51-year-old female patient with an eosinophilic liver abscess before (A-C) and after (D-F) albendazole treatment. The gadolinium-enhanced dynamic MR findings showed (A) low-signal intensity on unenhanced T1-weighted images, (B) high-signal intensity on unenhanced T2-weighted images, and (C) low-signal intensity in the portal phase. The previously identified 1.4 cm nodule (arrows) had completely disappeared 1 year after initial diagnosis (D, arterial phase; E, portal phase; F, equilibrium phase). |
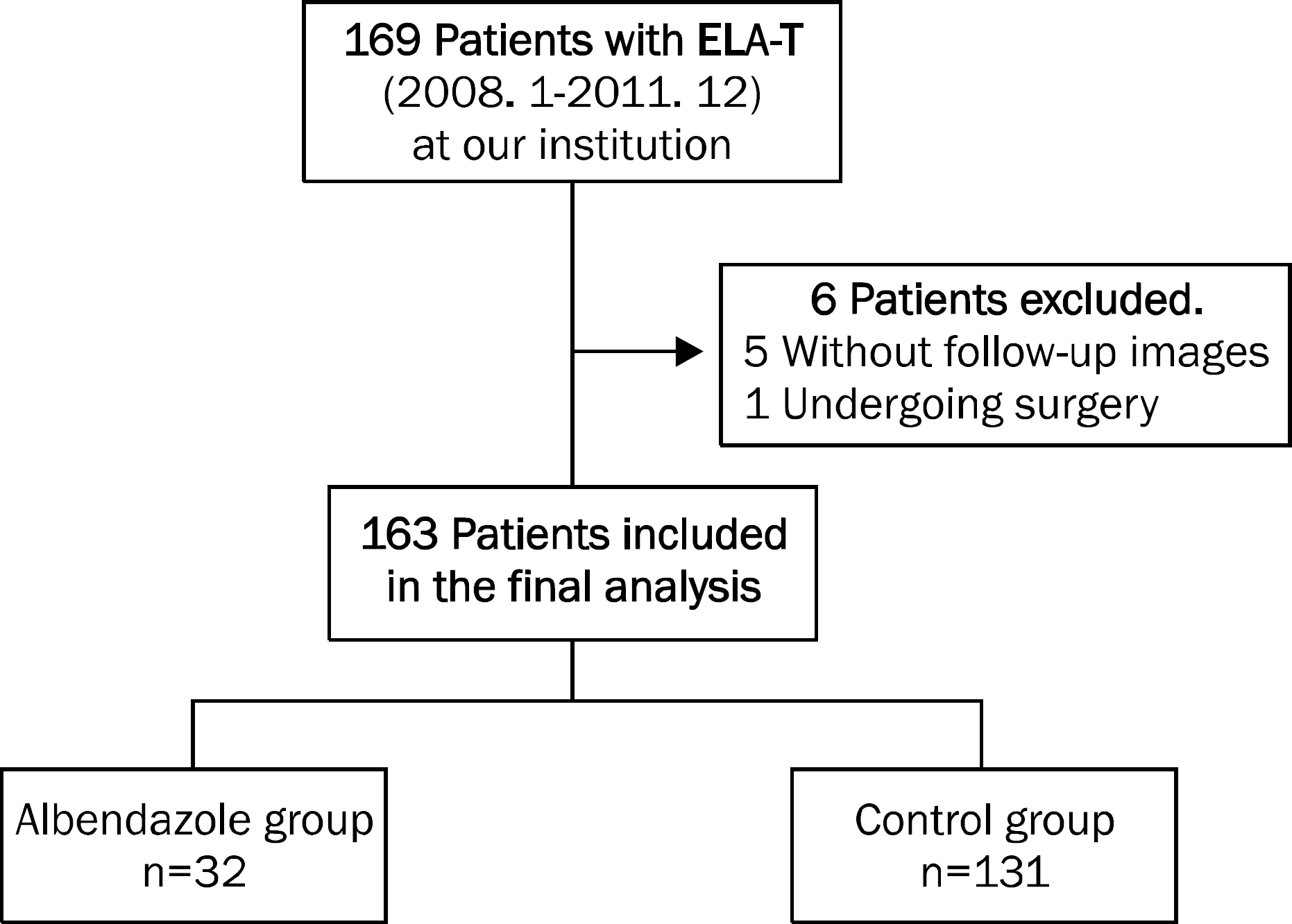 | Fig. 3.The enrolled patients in this study. A total of 169 patients with the eosinophilic liver abscesses with toxocariasis (ELA-T) were initially enrolled. Among them, six patients were excluded, and 163 patients were included in the final analysis. While 32 patients received albendazole treatment (albendazole group), 131 patients were followed up without medication (control group). |
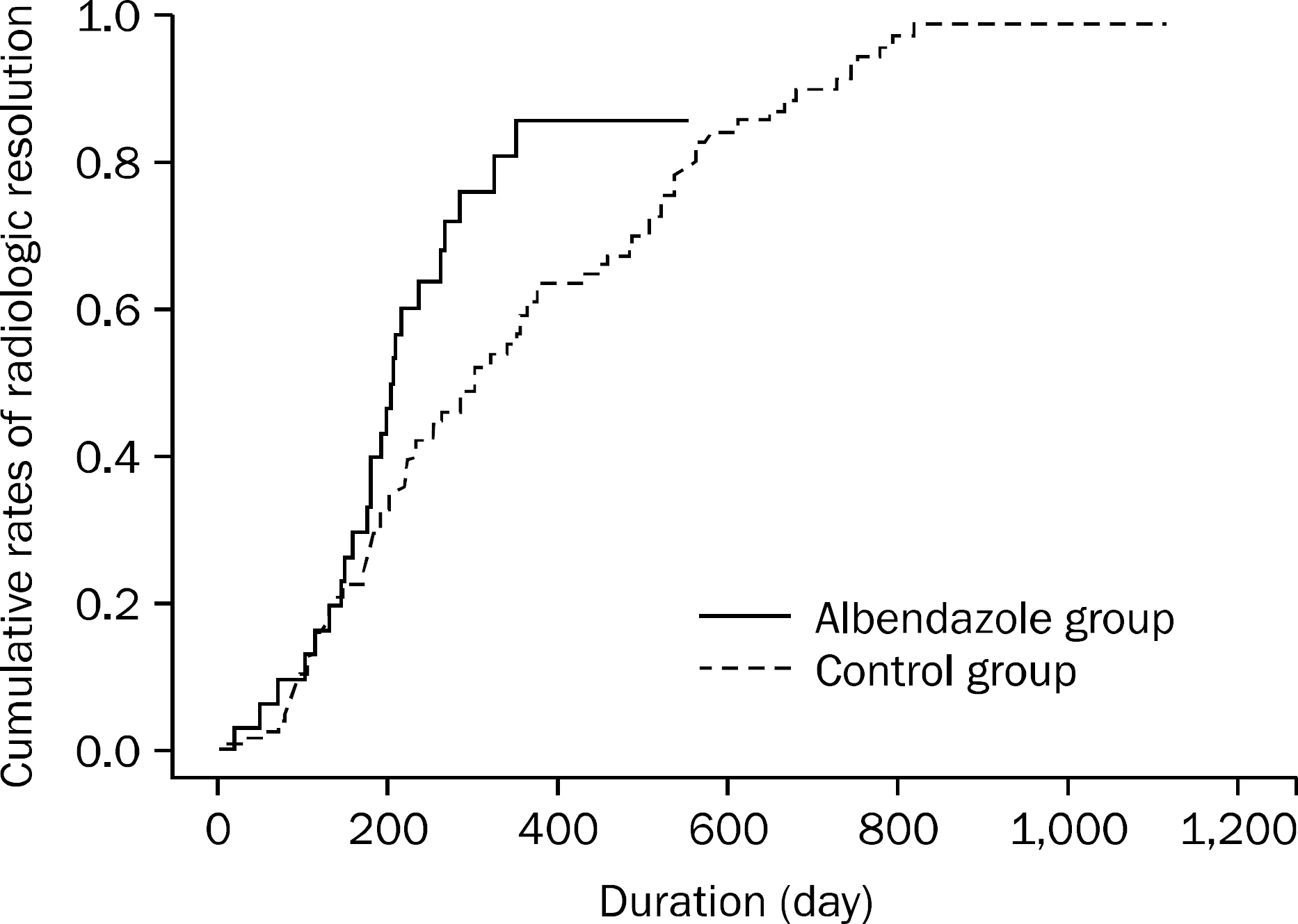 | Fig. 4.Cumulative rates of radiologic resolution by Kaplan-Meier analysis. Kaplan-Meier analysis showed that the median duration for achieving radiologic resolution was 207 days (range, 186–228 days) in the albendazole group, which was significantly shorter than the median duration for achieving radiologic resolution of 302 days in the control group (range, 224–380 days, p=0.023) |
Table 1.
Baseline Characteristics of Two Groups
Table 2.
Laboratory Findings of Two Groups




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download