Abstract
Background/Aims
Several clinical trials have revealed various advantages for probiotics in inflammatory bowel disease (IBD). The aim of this study was to further investigate the effects of probiotic yogurt consumption on gut microbiota in patients with this disease.
Methods
A total of 305 participants were divided into three groups; group A (IBD patients receiving probiotic yogurt; n=105), group B (IBD patients receiving placebo; n=105), and control group (healthy individuals receiving probiotic yogurt; n=95). Stool samples were collected both before and after 8 weeks of intervention; and population of Lactobacillus, Bifidobacterium and Bacteroides in the stool specimens was measured by Taqman real-time PCR method.
Results
By the end of the intervention, no significant variations in the mean weight and body mass index were observed between three groups (p>0.05). However, the mean numbers of Lactobacillus, Bifidobacterium, and Bacteroides in group A were significantly increased compared to group B (p<0.001, p<0.001, and p<0.01, respectively). There were also significant differences in the mean numbers of either of three bacteria between group A and the healthy control group; however, these differences between two groups were observed both at baseline and the end of the intervention.
References
1. Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007; 369:1627–1640.

2. Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006; 12(Suppl 1):S3–S9.

3. Karlinger K, Györke T, Makö E, Mester A, Tarján Z. The epidemiology and the pathogenesis of inflammatory bowel disease. Eur J Radiol. 2000; 35:154–167.

4. Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn's disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007; 5:1424–1429.

5. Orel R, Kamhi T, Vidmar G, Mamula P. Epidemiology of pediatric chronic inflammatory bowel disease in central and western Slovenia, 1994–2005. J Pediatr Gastroenterol Nutr. 2009; 48:579–586.

6. Lakatos PL, Fischer S, Lakatos L. Is the epidemiology of inflammatory bowel disease changing in Eastern Europe? Scand J Gastroenterol. 2006; 41:870–871.

7. Lovasz BD, Golovics PA, Vegh Z, Lakatos PL. New trends in inflammatory bowel disease epidemiology and disease course in Eastern Europe. Dig Liver Dis. 2013; 45:269–276.

8. Hou JK, Kramer JR, Richardson P, Mei M, El-Serag HB. The incidence and prevalence of inflammatory bowel disease among U.S. veterans: a national cohort study. Inflamm Bowel Dis. 2013; 19:1059–1064.
9. Thia KT, Loftus EV Jr, Sandborn WJ, Yang SK. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol. 2008; 103:3167–3182.

10. Vahedi H, Merat S, Momtahen S, et al. Epidemiologic characteristics of 500 patients with inflammatory bowel disease in Iran studied from 2004 through 2007. Arch Iran Med. 2009; 12:454–460.
11. Aghazadeh R, Zali MR, Bahari A, Amin K, Ghahghaie F, Firouzi F. Inflammatory bowel disease in Iran: a review of 457 cases. J Gastroenterol Hepatol. 2005; 20:1691–1695.

12. Shamir R. Nutrition and growth in inflammatory bowel disease. World Rev Nutr Diet. 2013; 106:156–161.

13. Gentschew L, Ferguson LR. Role of nutrition and microbiota in susceptibility to inflammatory bowel diseases. Mol Nutr Food Res. 2012; 56:524–535.

15. Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008; 134:577–594.

16. Quigley EM. Gut microbiota and the role of probiotics in therapy. Curr Opin Pharmacol. 2011; 11:593–603.

17. Iannitti T, Palmieri B. Therapeutical use of probiotic formulations in clinical practice. Clin Nutr. 2010; 29:701–725.

18. Asakura H, Suzuki K, Honma T. Recent advances in basic and clinical aspects of inflammatory bowel disease: which steps in the mucosal inflammation should we block for the treatment of inflammatory bowel disease? World J Gastroenterol. 2007; 13:2145–2149.

19. Mai V, Draganov PV. Recent advances and remaining gaps in our knowledge of associations between gut microbiota and human health. World J Gastroenterol. 2009; 15:81–85.

20. Steer T, Carpenter H, Tuohy K, Gibson GR. Perspectives on the role of the human gut microbiota and its modulation by pro- and prebiotics. Nutr Res Rev. 2000; 13:229–254.

21. Othman M, Agüero R, Lin HC. Alterations in intestinal microbial flora and human disease. Curr Opin Gastroenterol. 2008; 24:11–16.

22. Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995; 125:1401–1412.

23. O'Mahony L, Feeney M, O'Halloran S, et al. Probiotic impact on microbial flora, inflammation and tumour development in IL-10 knockout mice. Aliment Pharmacol Ther. 2001; 15:1219–1225.
24. Prisciandaro L, Geier M, Butler R, Cummins A, Howarth G. Probiotics and their derivatives as treatments for inflammatory bowel disease. Inflamm Bowel Dis. 2009; 15:1906–1914.

25. Meijer BJ, Dieleman LA. Probiotics in the treatment of human inflammatory bowel diseases: update 2011. J Clin Gastroenterol. 2011; 45(Suppl):S139–S144.
26. Brown AC, Valiere A. Probiotics and medical nutrition therapy. Nutr Clin Care. 2004; 7:56–68.
27. Pineda Mde L, Thompson SF, Summers K, de Leon F, Pope J, Reid G. A randomized, double-blinded, placebo-controlled pilot study of probiotics in active rheumatoid arthritis. Med Sci Monit. 2011; 17:CR347–CR354.

28. Cui HH, Chen CL, Wang JD, et al. Effects of probiotic on intestinal mucosa of patients with ulcerative colitis. World J Gastroenterol. 2004; 10:1521–1525.

29. Lorea Baroja M, Kirjavainen PV, Hekmat S, Reid G. Anti-inflammatory effects of probiotic yogurt in inflammatory bowel disease patients. Clin Exp Immunol. 2007; 149:470–479.

30. Veerappan GR, Betteridge J, Young PE. Probiotics for the treatment of inflammatory bowel disease. Curr Gastroenterol Rep. 2012; 14:324–333.

31. Malin M, Suomalainen H, Saxelin M, Isolauri E. Promotion of IgA immune response in patients with Crohn's disease by oral bac-teriotherapy with Lactobacillus GG. Ann Nutr Metab. 1996; 40:137–145.
32. Mach T. Clinical usefulness of probiotics in inflammatory bowel diseases. J Physiol Pharmacol. 2006; 57(Suppl 9):23–33.
33. Ishikawa H, Akedo I, Umesaki Y, Tanaka R, Imaoka A, Otani T. Randomized controlled trial of the effect of Bifidobacteria-fer-mented milk on ulcerative colitis. J Am Coll Nutr. 2003; 22:56–63.

34. Kato K, Mizuno S, Umesaki Y, et al. Randomized placebo-controlled trial assessing the effect of Bifidobacteria-fermented milk on active ulcerative colitis. Aliment Pharmacol Ther. 2004; 20:1133–1141.

35. Fedorak R, Demeria D. Probiotic bacteria in the prevention and the treatment of inflammatory bowel disease. Gastroenterol Clin North Am. 2012; 41:821–842.

36. Macfarlane S, Steed H, Macfarlane GT. Intestinal bacteria and inflammatory bowel disease. Crit Rev Clin Lab Sci. 2009; 46:25–54.

37. Kim E, Yune S, Ha JM, et al. Predictive factors of response to medical therapy in Crohn's disease patients with intestinal obstruction. Korean J Gastroenterol. 2013; 62:213–218.

38. Venturi A, Gionchetti P, Rizzello F, et al. Impact on the composition of the faecal flora by a new probiotic preparation: prelimi-nary data on maintenance treatment of patients with ulcerative colitis. Aliment Pharmacol Ther. 1999; 13:1103–1108.

39. García-Albiach R, Pozuelo de Felipe MJ, Angulo S, et al. Molecular analysis of yogurt containing Lactobacillus del-brueckii subsp. bulgaricus and Streptococcus thermophilus in human intestinal microbiota. Am J Clin Nutr. 2008; 87:91–96.

40. Tannock GW, Munro K, Harmsen HJ, Welling GW, Smart J, Gopal PK. Analysis of the fecal microflora of human subjects consuming a probiotic product containing Lactobacillus rhamnosus DR20. Appl Environ Microbiol. 2000; 66:2578–2588.
Fig. 1.
Flow chart of the present experiment. Group A, inflammatory bowel disease (IBD) patients receiving probiotic yogurt; Group B, IBD patients receiving placebo; Control group, healthy individuals receiving probiotic yogurt.
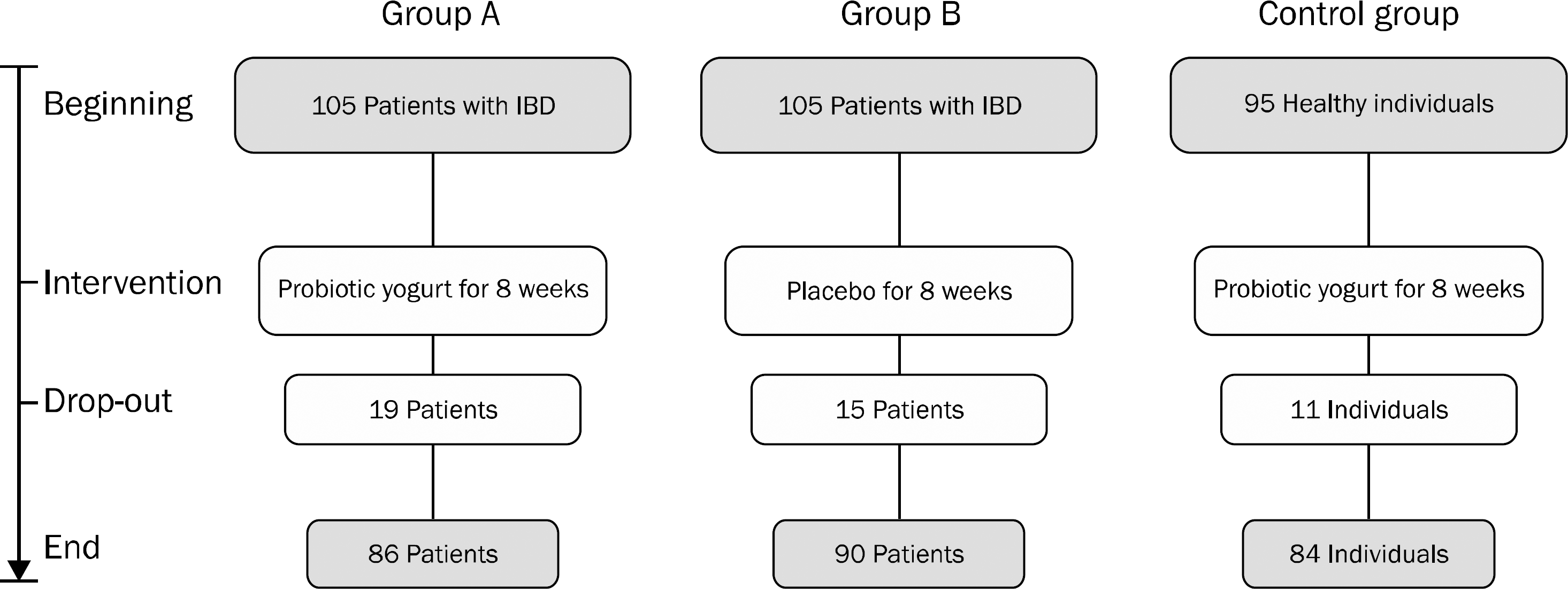
Table 1.
Primers and Probes Used for Detection of Each Bacterium a
Table 2.
Demographic and Clinical Features of the Patients with Inflammatory Bowel Disease (IBD)
Table 3.
Weight of the Participants before and after the Intervention
Table 4.
Stool Concentrations of Lactobacillus, Bifidobacterium and Bacteriodes in Group A and Group B before and after the Intervention
Table 5.
Stool Concentrations of Lactobacillus, Bifidobacterium and Bacteriodes in Group A and Control Group before and after the Intervention




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download