Abstract
Background/Aims
Gastric schwannoma (GS), a rare neurogenic mesenchymal tumor, is usually benign, slow-growing, and asymptomatic. However, GS is often misdiagnosed as gastrointestinal stromal tumors (GIST) on endoscopic and radiological examinations. The purpose of this study was to evaluate EUS characteristics of GS distinguished from GIST.
Methods
A total of 119 gastric subepithelial lesions, including 31 GSs and 88 GISTs, who were histologically identified and underwent EUS, were enrolled in this study. We evaluated the EUS characteristics, including location, size, gross morphology, mucosal lesion, layer of origin, border, echogenic pattern, marginal halo, and presence of an internal echoic lesion by retrospective review of the medical records.
Results
GS patients comprised nine males and 22 females, indicating female predominance. In the gross morphology according to Yamada's classification, type I was predominant in GS and type III was predominant in GIST. In location, GSs were predominantly located in the gastric body and GISTs were predominantly located in the cardia or fundus. The frequency of 4th layer origin and isoechogenicity as compared to the echogenicity of proper muscle layer was significantly more common in GS than GIST. Although not statistically significant, marginal halo was more frequent in GS than GIST. The presence of an internal echoic lesion was significantly more common in GIST than GS.
Go to : 
References
1. Papanikolaou IS, Triantafyllou K, Kourikou A, Rösch T. Endoscopic ultrasonography for gastric submucosal lesions. World J Gastrointest Endosc. 2011; 3:86–94.

2. Song JH, Kim JI, Kim HJ, et al. Endoscopic characteristics of upper gastrointestinal mesenchymal tumors originating from muscularis mucosa or muscularis propria. Korean J Gastroenterol. 2013; 62:92–96.

3. Hou YY, Tan YS, Xu JF, et al. Schwannoma of the gastrointestinal tract: a clinicopathological, immunohistochemical and ultra-structural study of 33 cases. Histopathology. 2006; 48:536–545.

4. Voltaggio L, Murray R, Lasota J, Miettinen M. Gastric schwannoma: a clinicopathologic study of 51 cases and critical review of the literature. Hum Pathol. 2012; 43:650–659.

5. Zhong DD, Wang CH, Xu JH, Chen MY, Cai JT. Endoscopic ultrasound features of gastric schwannomas with radiological correlation: a case series report. World J Gastroenterol. 2012; 18:7397–7401.

6. Jung MK, Jeon SW, Cho CM, et al. Gastric schwannomas: endosonographic characteristics. Abdom Imaging. 2008; 33:388–390.

7. Yamada T, Ichikawa H. X-ray diagnosis of elevated lesions of the stomach. Radiology. 1974; 110:79–83.

8. Daimaru Y, Kido H, Hashimoto H, Enjoji M. Benign schwannoma of the gastrointestinal tract: a clinicopathologic and immunohistochemical study. Hum Pathol. 1988; 19:257–264.

9. Kwon MS, Lee SS, Ahn GH. Schwannomas of the gastrointestinal tract: clinicopathological features of 12 cases including a case of esophageal tumor compared with those of gastrointestinal stromal tumors and leiomyomas of the gastrointestinal tract. Pathol Res Pract. 2002; 198:605–613.

10. Loffeld RJ, Balk TG, Oomen JL, van der Putten AB. Upper gastrointestinal bleeding due to a malignant Schwannoma of the stomach. Eur J Gastroenterol Hepatol. 1998; 10:159–162.
11. Gennatas CS, Exarhakos G, Kondi-Pafiti A, Kannas D, Athanassas G, Politi HD. Malignant schwannoma of the stomach in a patient with neurofibromatosis. Eur J Surg Oncol. 1988; 14:261–264.
12. Levy AD, Quiles AM, Miettinen M, Sobin LH. Gastrointestinal schwannomas: CT features with clinicopathologic correlation. AJR Am J Roentgenol. 2005; 184:797–802.

13. Hong HS, Ha HK, Won HJ, et al. Gastric schwannomas: radiological features with endoscopic and pathological correlation. Clin Radiol. 2008; 63:536–542.

14. Choi JW, Choi D, Kim KM, et al. Small submucosal tumors of the stomach: differentiation of gastric schwannoma from gastrointestinal stromal tumor with CT. Korean J Radiol. 2012; 13:425–433.

15. Okai T, Minamoto T, Ohtsubo K, et al. Endosonographic evaluation of c-kit-positive gastrointestinal stromal tumor. Abdom Imaging. 2003; 28:301–307.

16. Pidhorecky I, Cheney RT, Kraybill WG, Gibbs JF. Gastrointestinal stromal tumors: current diagnosis, biologic behavior, and management. Ann Surg Oncol. 2000; 7:705–712.

17. Miettinen M, Sobin LH, Sarlomo-Rikala M. Immunohistochemical spectrum of GISTs at different sites and their differential diagnosis with a reference to CD117 (KIT). Mod Pathol. 2000; 13:1134–1142.

18. Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, Miettinen M. CD117: a sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod Pathol. 1998; 11:728–734.
19. Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005; 29:52–68.
Go to : 
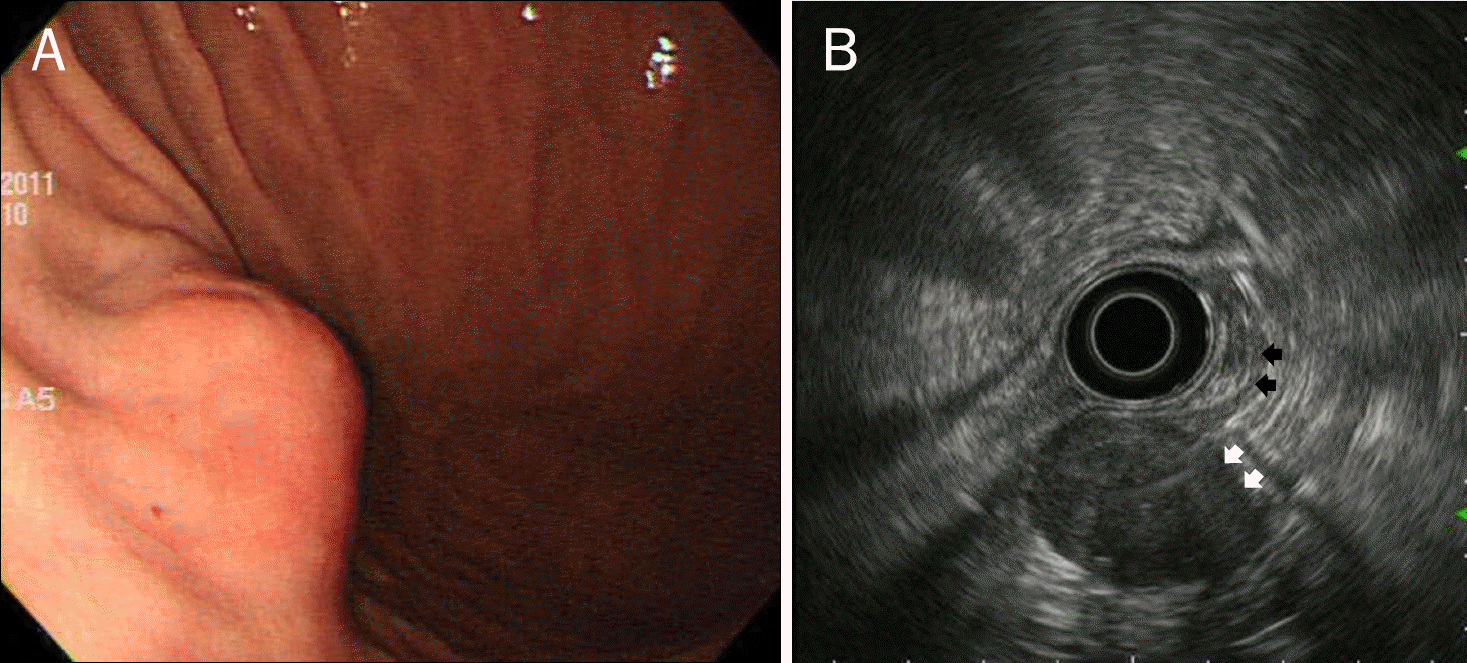 | Fig. 1.Endoscopic (A) and endosonographic (B) findings in a 59-year-old woman with a gastric schwannoma. (A) Endoscopy shows a submucosal elevated lesion with type I morphology according to Yamada's classification in the greater curvature of the body. (B) On EUS, the mass is homogeneous and its echogenicity is similar to that of the normal proper muscle layer (black arrows). It measures 32.0×21.0 mm in size and a marginal halo (white arrows) is observed. |
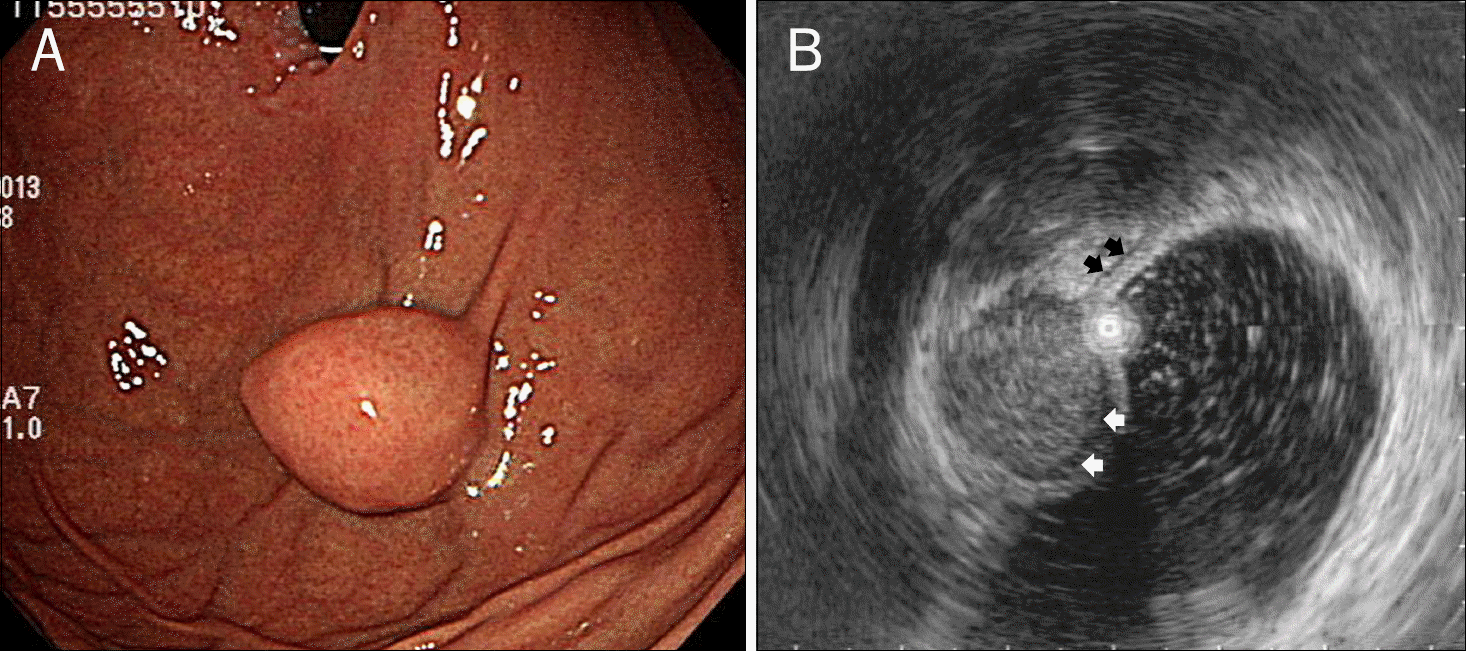 | Fig. 2.Endoscopic (A) and endosonographic (B) findings in a 66-year-old woman with a gastrointestinal stromal tumor. (A) Endoscopy shows a submucosal elevated lesion with type III morphology according to Yamada's classification in the cardia. (B) On EUS, the mass is homogeneous and its echogenicity is higher than that of the normal proper muscle layer (black arrows). It measures 20.0×16.0 mm in size and a marginal halo (white arrows) is observed. |
Table 1.
Baseline Characteristics of the Patients with GSs and GISTs of the Stomach
Table 2.
Endoscopic Features of the Patients with GSs and GISTs of the Stomach
| Variable | GS (n=31) | GIST (n=88) p-value |
|---|---|---|
| Shape | 0.062 | |
| Round to ovoid | 31 (100) | 78 (88.6) |
| Lobulated | 0 (0) | 10 (11.4) |
| Mucosal lesion | 1.000 | |
| Present | 5 (16.1) | 14 (15.9) |
| Absent | 26 (83.9) | 74 (84.1) |
| Morphology (Yamada's classification) a | 0.030 | |
| Type I | 16 (51.6) | 26 (29.5) |
| Type II | 12 (38.7) | 35 (39.8) |
| Type III | 3 (9.7) | 27 (30.7) |
| Type IV | 0 (0) | 0 (0) |
| Location | 0.001 | |
| Body, GC | 19 (61.3) | 21 (23.9) |
| Body, LC | 9 (29.0) | 24 (27.3) |
| Cardia/fundus | 1 (3.2) | 37 (42.0) |
| Antrum | 2 (6.5) | 6 (6.8) |
a Yamada type I is elevated, with an indistinct border; Type II is elevated with a distinct border at the base but no notch; Type III is elevated, but no peduncle; Type IV is pedunculated and elevated. GS, gastric schwannoma; GIST, gastrointestinal stromal tumor; GC, greater curvature; LC, lesser curvature.
Table 3.
Endoscopic Ultrasonography Features of the Patients with GSs and GISTs of the Stomach




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download