Abstract
Background/Aims
The risk of gastrointestinal (GI) bleeding with dabigatran when compared to warfarin has been controversial in the literature. The aim of our study was to assess this risk with the use of dabigatran.
Methods
We examined the medical records of patients who were started on dabigatran or warfarin from October 2010 to October 2012. The study was conducted in two hospitals.
Results
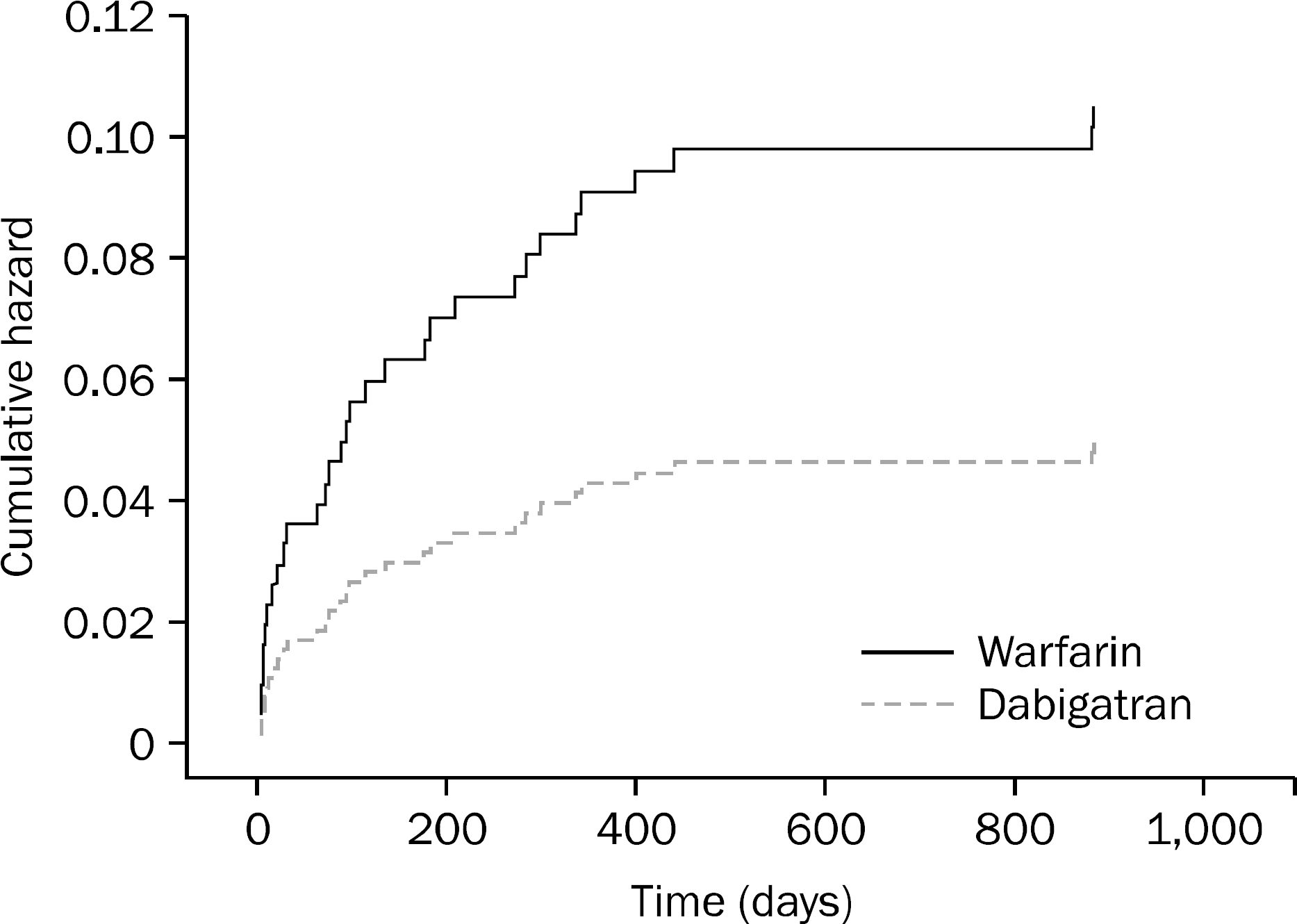
A total of 417 patients were included (208 dabigatran vs. 209 warfarin). GI bleeding occurred in 10 patients (4.8%) in the dabigatran group compared to 21 patients (10.1%) in the warfarin group (p=0.0375). Multivariate analysis showed that patients who were on dabigatran for ≤100 days had a higher incidence of GI bleeding than those who were on it for >100 days (p=0.0007). The odds of GI bleeding in patients who were on dabigatran for ≤100 days was 8.2 times higher compared to those who were on the drug for >100 days. The incidence of GI bleeding in patients >65 years old was higher than in those <65 years old (p=0.0453, OR=3). History of previous GI bleeding was another risk factor for GI bleeding in the dabigatran group (p=0.036, OR=6.3). The lower GI tract was the most common site for GI bleeding in the dabigatran group (80.0% vs. 38.1%, p=0.014).
Go to : 
References
1. Connolly SJ, Ezekowitz MD, Yusuf S, et al. RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361:1139–1151.

2. Schulman S, Kearon C, Kakkar AK, et al. RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009; 361:2342–2352.

3. Stangier J, Rathgen K, Stähle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007; 64:292–303.

4. Stangier J, Stähle H, Rathgen K, Fuhr R. Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin Pharmacokinet. 2008; 47:47–59.
5. Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal im-pairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet. 2010; 49:259–268.
6. Walenga JM, Adiguzel C. Drug and dietary interactions of the new and emerging oral anticoagulants. Int J Clin Pract. 2010; 64:956–967.

7. Dans AL, Connolly SJ, Wallentin L, et al. Concomitant use of antiplatelet therapy with dabigatran or warfarin in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial. Circulation. 2013; 127:634–640.

8. Eikelboom JW, Wallentin L, Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation. 2011; 123:2363–2372.
9. Larsen TB, Rasmussen LH, Skj⊘th F, et al. Efficacy and safety of dabigatran etexilate and warfarin in “real-world” patients with atrial fibrillation: a prospective nationwide cohort study. J Am Coll Cardiol. 2013; 61:2264–2273.
10. Eriksson BI, Dahl OE, Büller HR, et al. BISTRO II Study Group. A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: the BISTRO II randomized trial. J Thromb Haemost. 2005; 3:103–111.

11. Connolly SJ, Pogue J, Eikelboom J, et al. ACTIVE W Investigators. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008; 118:2029–2037.

12. White HD, Gruber M, Feyzi J, et al. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: results from SPORTIF III and V. Arch Intern Med. 2007; 167:239–245.
13. Jones M, McEwan P, Morgan CL, Peters JR, Goodfellow J, Currie CJ. Evaluation of the pattern of treatment, level of anticoagulation control, and outcome of treatment with warfarin in patients with non-valvar atrial fibrillation: a record linkage study in a large British population. Heart. 2005; 91:472–477.

14. Wallentin L, Yusuf S, Ezekowitz MD, et al. RE-LY investigators. Efficacy and safety of dabigatran compared with warfarin at dif-ferent levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet. 2010; 376:975–983.

15. Samsa GP, Matchar DB, Goldstein LB, et al. Quality of anticoagulation management among patients with atrial fibrillation: results of a review of medical records from 2 communities. Arch Intern Med. 2000; 160:967–973.
16. Sarawate C, Sikirica MV, Willey VJ, Bullano MF, Hauch O. Monitoring anticoagulation in atrial fibrillation. J Thromb Thrombolysis. 2006; 21:191–198.

17. McBride D, Brüggenjürgen B, Roll S, Willich SN. Anticoagulation treatment for the reduction of stroke in atrial fibrillation: a cohort study to examine the gap between guidelines and routine medical practice. J Thromb Thrombolysis. 2007; 24:65–72.

Go to : 
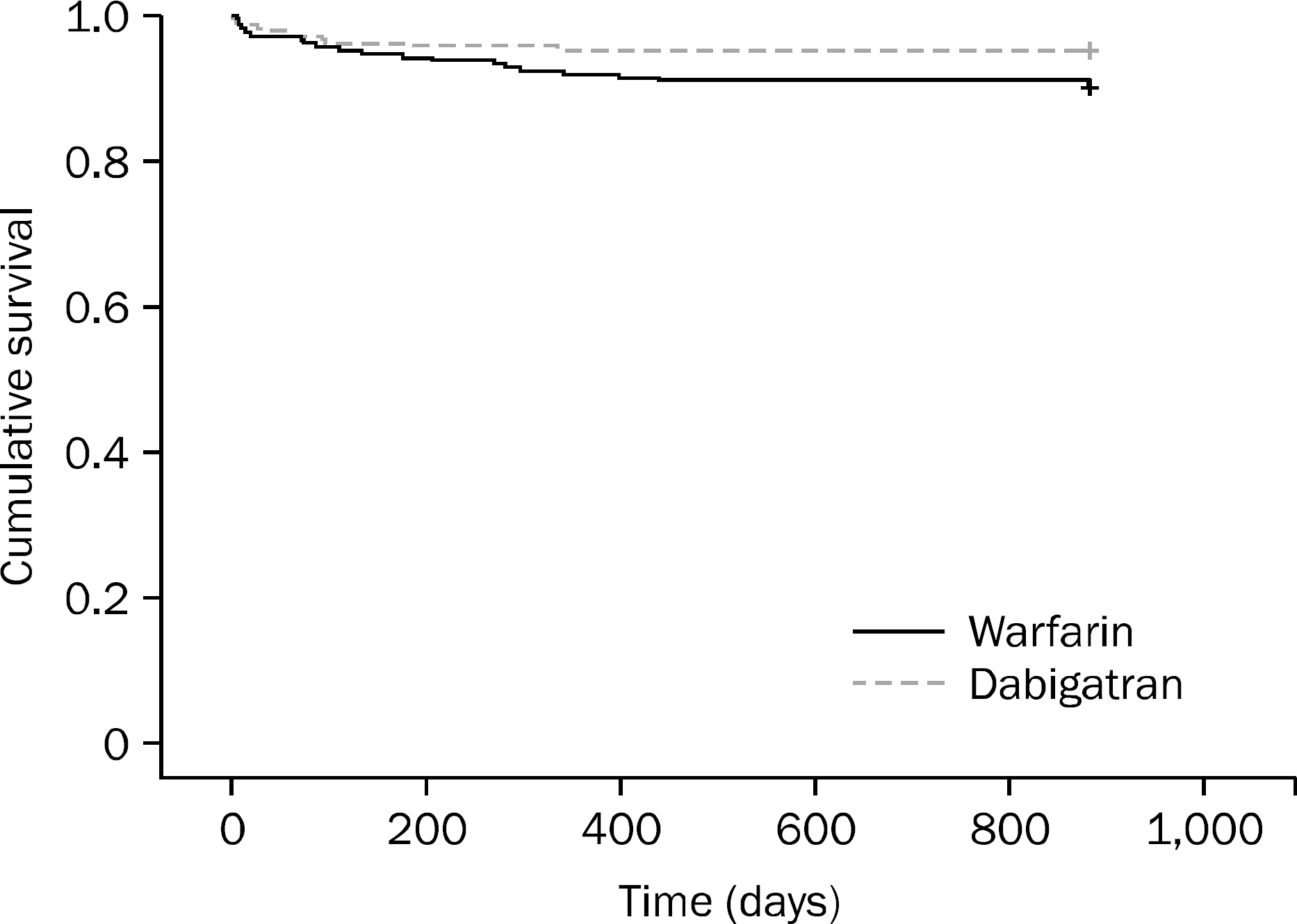
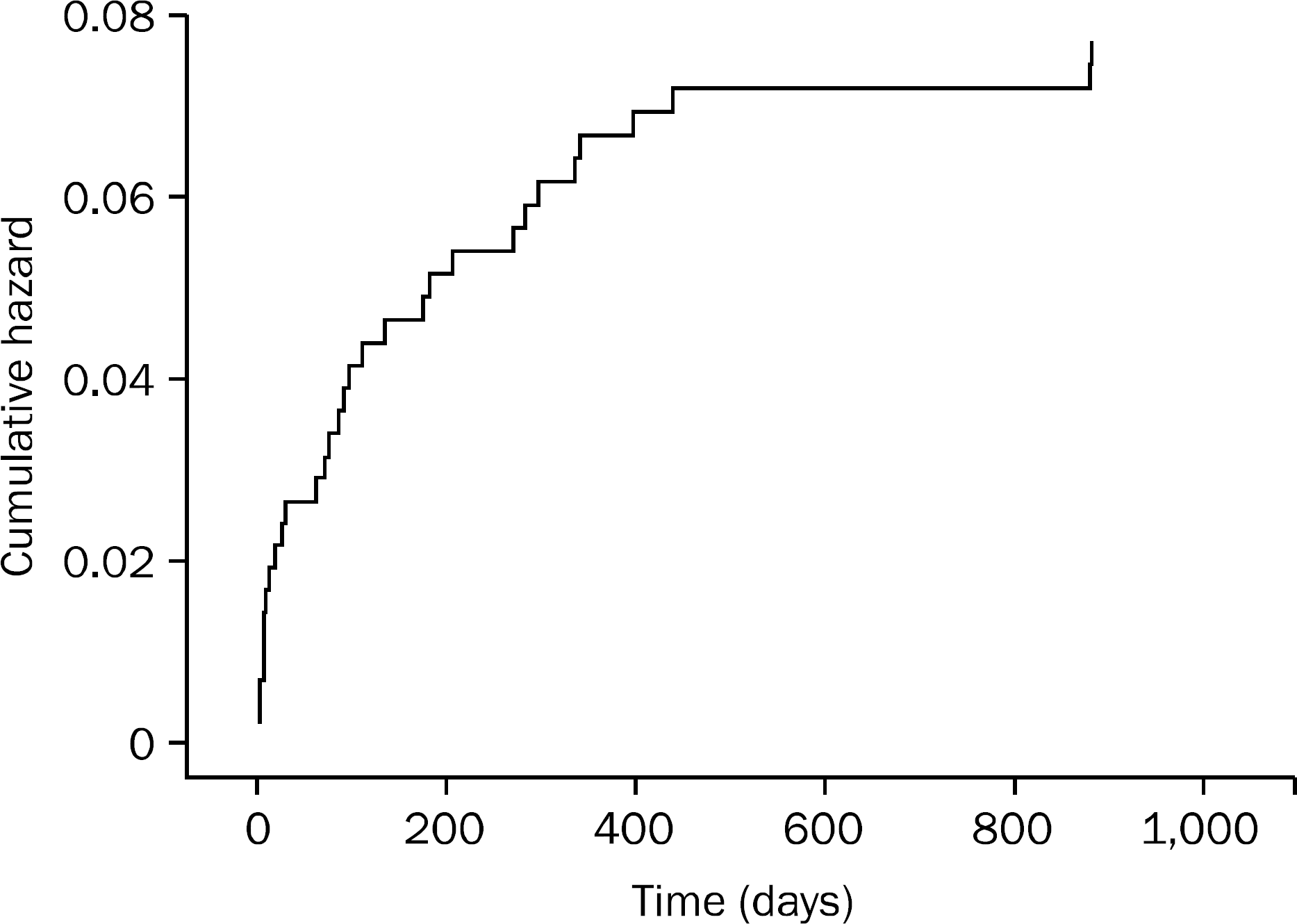 | Fig. 1.Cumulative gastrointestinal bleeding free survival in total subjects, including both warfarin and dabigatran groups. |
Table 1.
Patients' Characteristics in Both Groups
| Characteristic | Dabigatran group (n=208) | Warfarin group (n=209) | p-value |
|---|---|---|---|
| Mean age (yr) | 72.72 | 71.83 | 0.474 |
| Age >85 yr | 35 (16.8) | 29 (13.9) | 0.403 |
| Sex (female) | 104 (50.0) | 113 (54.1) | 0.406 |
| Race (Caucasian) | 190 (91.4) | 198 (94.7) | 0.130 |
| Indication for drug: atrial fibrillation | 206 (99.0) | 149 (71.3) | 0.001 a |
| Dose (mg) | |||
| 150 | 166 (79.8) | NA | NA |
| 75 | 42 (20.2) | NA | NA |
| Mean duration being on drug (day) | 289.66 | 355.86 | 0.004 a |
| Duration ≤100 days | 47 (22.6) | 46 (22.0) | 0.886 |
| Drug was discontinued | 39 (18.8) | 51 (24.4) | 0.161 |
| Concomitant use with | |||
| Aspirin | 100 (48.1) | 101 (48.3) | 0.960 |
| Thienopyridines | 26 (12.5) | 33 (15.8) | 0.929 |
| Dual antiplatelet agents | 16 (7.7) | 19 (9.1) | 0.607 |
| NSAIDs | 14 (6.7) | 14 (6.7) | 0.990 |
| GFR ≤30 mL/min/1.73 m2 | 10 (4.8) | 8 (3.8) | 0.622 |
| Previous GI bleeding | 11 (5.3) | 19 (9.1) | 0.053 |
Table 2.
Characteristics of Patients with Gastrointestinal (GI) Bleeding in Both Groups
| Characteristic | Dabigatran group | Warfarin group | p-value |
|---|---|---|---|
| GI bleeding event | 10 (4.8) | 21 (10.1) | 0.038 a |
| Mean age (yr) | 79.20 | 75.86 | 0.441 |
| Age >85 yr | 3 (30.0) | 4 (19.1) | 0.495 |
| Sex (female) | 8 (80.0) | 13 (61.9) | 0.428 |
| Race (Caucasian) | 8 (80.0) | 21 (100) | 0.097 |
| Indication for drug: atrial fibrillation | 10 (100) | 17 (81.0) | 0.277 |
| Dose (mg) | |||
| 150 | 7 (70.0) | NA | NA |
| 75 | 3 (30.0) | NA | NA |
| Mean duration being on drug (day) | 108.50 | 188.86 | 0.189 |
| Duration ≤100 days | 8 (80.0) | 9 (42.8) | 0.052 |
| Concomitant use with | |||
| Aspirin | 4 (40.0) | 11 (52.4) | 0.704 |
| Thienopyridines | 1 (10.0) | 7 (33.3) | 0.165 |
| Dual antiplatelet agents | 1 (10) | 6 (28.6) | 0.248 |
| NSAIDs | 0 | 0 | NA |
| GFR≤30 mL/min/1.73 m2 | 2 (20.0) | 2 (9.5) | 0.416 |
| Previous GI Bleeding | 2 (20.0) | 2 (9.5) | 0.416 |
| Upper GI tract | 1 (10.0) | 9 (42.8) | 0.067 |
| Lower GI tract | 8 (80.0) | 8 (38.1) | 0.014 a |
| Occult obscure GI bleeding | 1 (10.0) | 4 (19.0) | 1.00 |
| Death related to GI bleeding | 0 | 0 | NA |
Table 3.
Multivariate Analysis for the Dabigatran Group
| Variable | Adjusted OR (95% CI) | p-value |
|---|---|---|
| Age >65 years | 2.989 (1.785-24.782) | 0.0453 a |
| Sex (female) | 2.732 (0.514-14.509) | 0.238 |
| Race (Caucasian) | 0.612 (1.33-2.816) | 0.528 |
| Duration <100 days | 8.176 (1.993-38.547) | 0.0007 a |
| Concomitant with | ||
| Aspirin | 1.739 (1.64-4.781) | 0.657 |
| Thienopyridines | 1.051 (0.752-7.438) | 0.279 |
| Dual antiplatelet | 0.856 (0.675-9.409) | 0.492 |
| NSAIDs | 1.297 (1.824-5.721) | 0.573 |
| GFR≤30 mL/min/1.73 m2 | 4.534 (0.682-30.138) | 0.118 |
| Previous GI bleeding | 6.284 (0.612-28.591) | 0.036 a |
Table 4.
Source of Gastrointestinal (GI) Bleeding in Both Groups
| Source of GI bleeding | Dabigatran group (n) | Warfarin group (n) |
|---|---|---|
| Upper GI tract | Severe hemorrhagic gastritis with pneumatosis of gastric wall and portal vein air on CT scan (1) | PUD (3) |
| AVM in stomach/duodenum (3) | ||
| Bleeding from sphinctertomy site that performed recently (1) | ||
| Scopes were not performed a (2) | ||
| Lower GI tract | Colorectal cancer (2) | Colorectal cancer (2) |
| Ischemic colitis (2) | Internal hemorrhoids (1) | |
| Internal hemorrhoids/diverticulosis (1) | Diverticulosis (1) | |
| Scopes were not performed because patients’ refusal b (3) | Large cecal polyp (1) | |
| Sigmoid ulcer (1) | ||
| Anal fissure (1) | ||
| Scopes were not performed because patients’ refusal b (1) | ||
| Occult obscure GI bleeding | EGD/colonoscopy/push enteroscopy were negative (1) | EGD/colonoscopy/push enteroscopy were negative (4) |
Table 5.
Secondary Aims of Our Study
| Event | Dabigatran group (n=208) | Warfarin group (n=209) | p-value |
|---|---|---|---|
| Major bleeding other than GI bleeding | 3 (1.4) | 9 (4.3) | 0.080 |
| ICH | 0 (0) | 1 (0.5) | 1.00 |
| Stroke or TIA | 4 (1.9) | 6 (2.9) | 0.751 |
| DVT or PE | 0 (0) | 3 (1.4) | 0.083 |
| ACS | 4 (1.9) | 17 (8.1) | 0.006 a |
| Death | 7 (3.4) | 15 (7.2) | 0.082 |
Table 6.
Comparison of the Results of Our Studies with Other Studies
| Study | Indication | Durationa (mo) | Group (patient) | Mean age (yr) | Sex (male, %) | Race (Caucasian, %) | Drug discontinued c (%) | GI bleeding | Major bleeding d (%) | ICH (%) | Stroke or TIA (%) | DVT/PE (%) | ACS (%) | Death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RE-LY | AF | 24 | Dabigatran b (6,076) | 71.5 | 63.20 | NA | 21.20 | 1.51%/yr | 3.11/yr | 0.30/yr | 1.01/yr | 0.15/yr | 0.74/yr | 3.64/yr |
| Warfarin (6,022) | 71.6 | 63.30 | NA | 16.60 | 1.02%/yr (p−0.001) | 3.36/yr (p=0.31) | 0.74/yr (p<0.001) | )1.57/yr (p<0.001) | 0.09/yr (p=0.21) | 0.53/yr (p=0.048) | 4.13/yr (p=0.051) | |||
| RE-COVER | DVT/PE | 6 | Dabigatran b (1,274) | 55 | 58 | 95.20 | 16 | 4.20% | 1.60 | 0 | NA | 2.40 | 0.40 | 1.60 |
| Warfarin (1,265) | 54.4 | 58.90 | 94.40 | 14.50 | 2.80% | 1.90 | 0.23 | NA | 2.1 (p<0.001) | 0.2 (p=0.73) | 1.70 | |||
| Danish registry | AF | 17 | Dabigatran b (2,239) | 67.4 | 61.50 | NA | NA | 1.50% | 2.20 | 0.10 | 3.50 | 0.20 | 0.90 | 3 |
| Warfarin (8,936) | 69.7 | 59.80 | NA | NA | 1.5% (p=0.26) | 2.9/yr (p=0.15) | 0.70 | 3 (p=0.05) | 0.50 | 1.9 (p=0.06) | 4.7 (p=0.03) | |||
| Mini-Sentinel initiative FDA | 15 | Dabigatran (12,195) | NA | NA | NA | NA | 1.6 per 100,00 days at risk | NA 00 | 0.90 | NA | NA | NA | NA | |
| Warfarin (119,940) | NA | NA | NA | NA | 3.1 per 100,00 days at risk | 00 NA | 1.90 | NA | NA | NA | NA | |||
| Our study | 24 | Dabigtran (208) | 72.72 | 50 | 91.40 | 18.80 | 4.80% | 6.30 | 0 | 1.90 | 0 | 1.90 | 3.40 | |
| Warfarin (209) | 71.83 | 45.90 | 94.70 | 24.40 | 10.1% (p=0.0375) | 14.40 | 0.50 | 2.9 (p=0.751) | 1.4 (p=0.083) | 8.1 (p=0.006) | 7.2 (p=0.082) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download