Abstract
Background/Aims
Hepatitis C genotypes 1 and 2 are widely distributed globally. In contrast, genotype 6 is found mainly in Southeast Asia, while genotype 6 is rare in Korea. This study aims to investigate the prevalence, risk factors and clinical characteristics of patients with genotype 6 chronic hepatitis C.
Methods
We retrospectively identified 133 HCV-infected patients who underwent HCV genotype analysis between January 2012 and December 2012, and analyzed the prevalence, risk factors and clinical characteristics of patients diagnosed with genotype 6 chronic hepatitis C.
Results
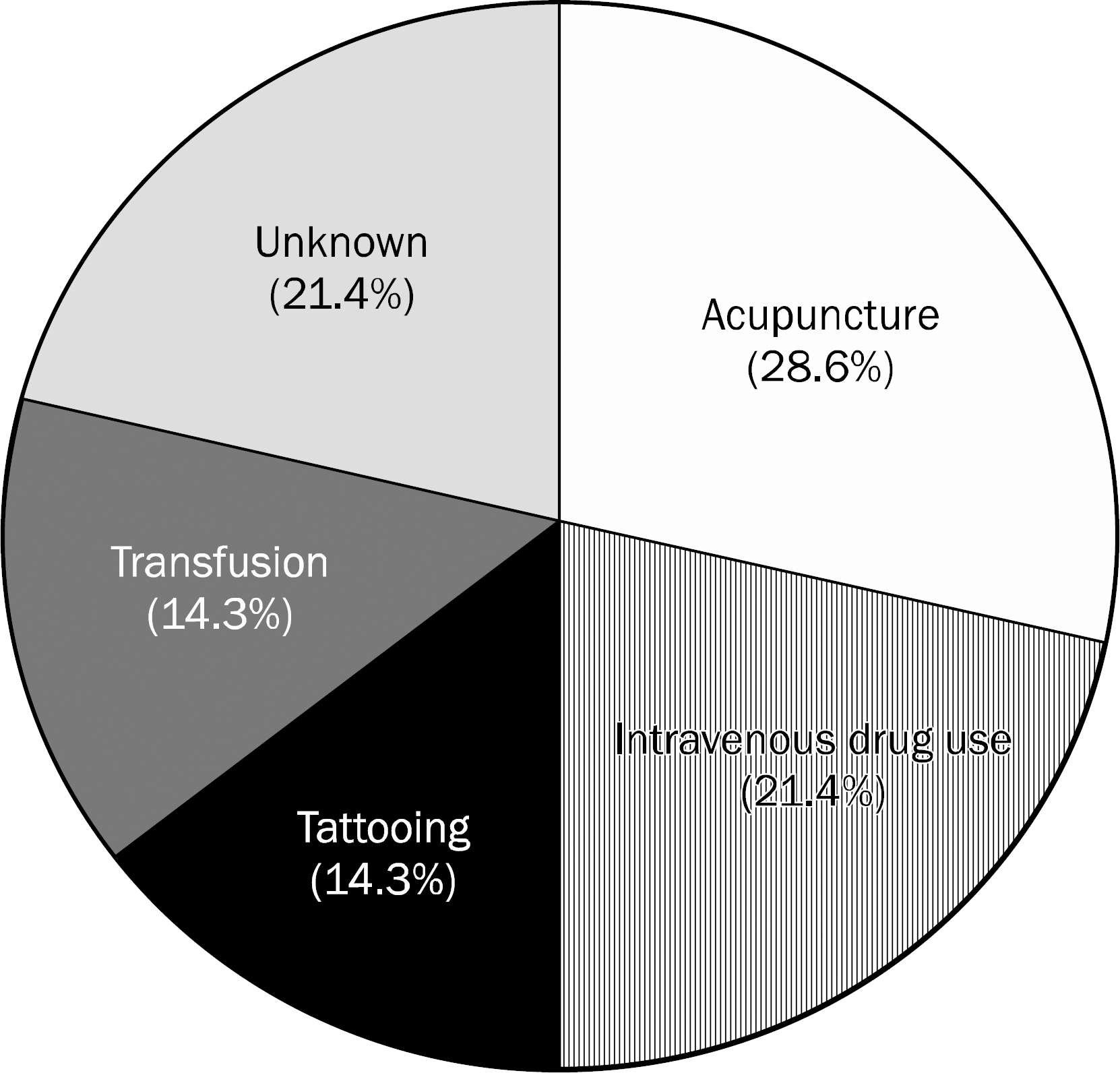
Among 133 patients, 53 patients (39.8%) were infected with genotype 1, 62 patients (46.6%) with genotype 2, 2 patients (1.5%) with genotype 3, 14 patients (10.5%) with genotype 6, and 2 patients (1.5%) with mixed genotypes (genotype 1 and 6). The risk factors associated with genotype 6 were acupuncture (n=4, 28.6%), intravenous drug use (n=3, 21.4%), tattoo (n=2, 14.3%), and transfusion (n=2, 14.3%). Of the 14 patients with genotype 6, 6 patients were treated with pegylated interferon and ribavirin. Five patients had reached the end of treatment. All patients reaching end of treatment for genotype 6 showed early virological response and sustained virological response.
Conclusions
The prevalence of genotype 6 is 10.5% and mixed infections of genotype 1 and 6 are 1.5% in patients with chronic hepatitis C. A major potential risk factor is intravenous drug use and the treatment response rate to pegylated interferon plus ribavirin is high in patients with genotype 6 chronic hepatitis C. Large scale multicenter studies are needed.
Go to : 
References
1. Ghany MG, Strader DB, Thomas DL, Seeff LB. American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009; 49:1335–1374.

3. Esteban JI, Sauleda S, Quer J. The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol. 2008; 48:148–162.

4. Rustgi VK. The epidemiology of hepatitis C infection in the United States. J Gastroenterol. 2007; 42:513–521.

5. Zein NN. Clinical significance of hepatitis C virus genotypes. Clin Microbiol Rev. 2000; 13:223–235.

6. Seong MH, Kil H, Kim JY, et al. Clinical and epidemiological characteristics of Korean patients with hepatitis C virus genotype 6. Clin Mol Hepatol. 2013; 19:45–50.

7. Hnatyszyn HJ. Chronic hepatitis C and genotyping: the clinical significance of determining HCV genotypes. Antivir Ther. 2005; 10:1–11.
8. Lee HS, Kim JK, Cheong JY, et al. Prediction of compensated liver cirrhosis by ultrasonography and routine blood tests in patients with chronic viral hepatitis. Korean J Hepatol. 2010; 16:369–375.

9. Korean Liver Cancer Study Group and National Cancer Center, Korea. Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol. 2009; 15:391–423.
10. Oh HB, Kim SO, Cha CH, et al. Identification of hepatitis C virus genotype 6 in Korean patients by analysis of 5' untranslated region using a matrix assisted laser desorption/ionization time of flight-based assay, restriction fragment mass polymorphism. J Med Virol. 2008; 80:1712–1719.

11. Schreier E, Roggendorf M, Driesel G, Hohne M, Viazov S. Genotypes of hepatitis C virus isolates from different parts of the world. Arch Virol Suppl. 1996; 11:185–193.

12. Davidson F, Simmonds P, Ferguson JC, et al. Survey of major genotypes and subtypes of hepatitis C virus using RFLP of se-quences amplified from the 5' non-coding region. J Gen Virol. 1995; 76:1197–1204.

13. Mellor J, Walsh EA, Prescott LE, et al. Survey of type 6 group variants of hepatitis C virus in Southeast Asia by using a core-based genotyping assay. J Clin Microbiol. 1996; 34:417–423.

14. Chinchai T, Labout J, Noppornpanth S, et al. Comparative study of different methods to genotype hepatitis C virus type 6 variants. J Virol Methods. 2003; 109:195–201.

15. Dev AT, McCaw R, Sundararajan V, Bowden S, Sievert W. Southeast Asian patients with chronic hepatitis C: the impact of novel genotypes and race on treatment outcome. Hepatology. 2002; 36:1259–1265.

16. Wong DA, Tong LK, Lim W. High prevalence of hepatitis C virus genotype 6 among certain risk groups in Hong Kong. Eur J Epidemiol. 1998; 14:421–426.
17. Akkarathamrongsin S, Praianantathavorn K, Hacharoen N, et al. Geographic distribution of hepatitis C virus genotype 6 subtypes in Thailand. J Med Virol. 2010; 82:257–262.

18. Zhang Z, Yao Y, Wu W, et al. Hepatitis C virus genotype diversity among intravenous drug users in Yunnan Province, Southwestern China. PLoS One. 2013; 8:e82598.

19. Kim JY, Cho J, Hwang SH, et al. Behavioral and health-care-associated risk factors for chronic hepatitis C virus infection in Korea. J Korean Med Sci. 2012; 27:1371–1377.

20. Shin HR, Kim JY, Ohno T, et al. Prevalence and risk factors of hepatitis C virus infection among Koreans in rural area of Korea. Hepatol Res. 2000; 17:185–196.

21. Shin HR, Kim JY, Kim JI, et al. Hepatitis B and C virus prevalence in a rural area of South Korea: the role of acupuncture. Br J Cancer. 2002; 87:314–318.

22. Nguyen NH, VuTien P, Garcia RT, et al. Response to pegylated interferon and ribavirin in Asian American patients with chronic hepatitis C genotypes 1 vs 2/3 vs 6. J Viral Hepat. 2010; 17:691–697.

23. Nguyen MH, Keeffe EB. Prevalence and treatment of hepatitis C virus genotypes 4, 5, and 6. Clin Gastroenterol Hepatol. 2005; 3(10 Suppl 2):S97–S101.

24. Tangkijvanich P, Komolmit P, Mahachai V, Poovorawan K, Akkarathamrongsin S, Poovorawan Y. Response-guided therapy for patients with hepatitis C virus genotype 6 infection: a pilot study. J Viral Hepat. 2012; 19:423–430.

25. Bunchorntavakul C, Chavalitdhamrong D, Tanwandee T. Hepatitis C genotype 6: a concise review and response-guided therapy proposal. World J Hepatol. 2013; 5:496–504.

26. Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines: management of hepatitis C. Clin Mol Hepatol. 2014; 20:89–136.
27. Lam KD, Trinh HN, Do ST, et al. Randomized controlled trial of pegylated interferon-alfa 2a and ribavirin in treatment-naive chronic hepatitis C genotype 6. Hepatology. 2010; 52:1573–1580.

28. Thu Thuy PT, Bunchorntavakul C, Tan Dat H, Rajender Reddy K. A randomized trial of 48 versus 24 weeks of combination pegylated interferon and ribavirin therapy in genotype 6 chronic hepatitis C. J Hepatol. 2012; 56:1012–1018.

29. Nguyen MH, Trinh HN, Garcia R, Nguyen G, Lam KD, Keeffe EB. Higher rate of sustained virologic response in chronic hepatitis C genotype 6 treated with 48 weeks versus 24 weeks of peginterferon plus ribavirin. Am J Gastroenterol. 2008; 103:1131–1135.

30. Recommendations for testing, managing, and treating hepatitis C. [Internet]. Alexandria (VA): American Association for the Study of Liver Diseases [cited 2015 Feb 10]. Available from:. http://www.hcvguidelines.org/.
Go to : 
Table 1.
Clinical Characteristics of Patients according to Genotype
Table 2.
Treatment Results of Patients with Genotype 6 Chronic Hepatitis C




 PDF
PDF ePub
ePub Citation
Citation Print
Print


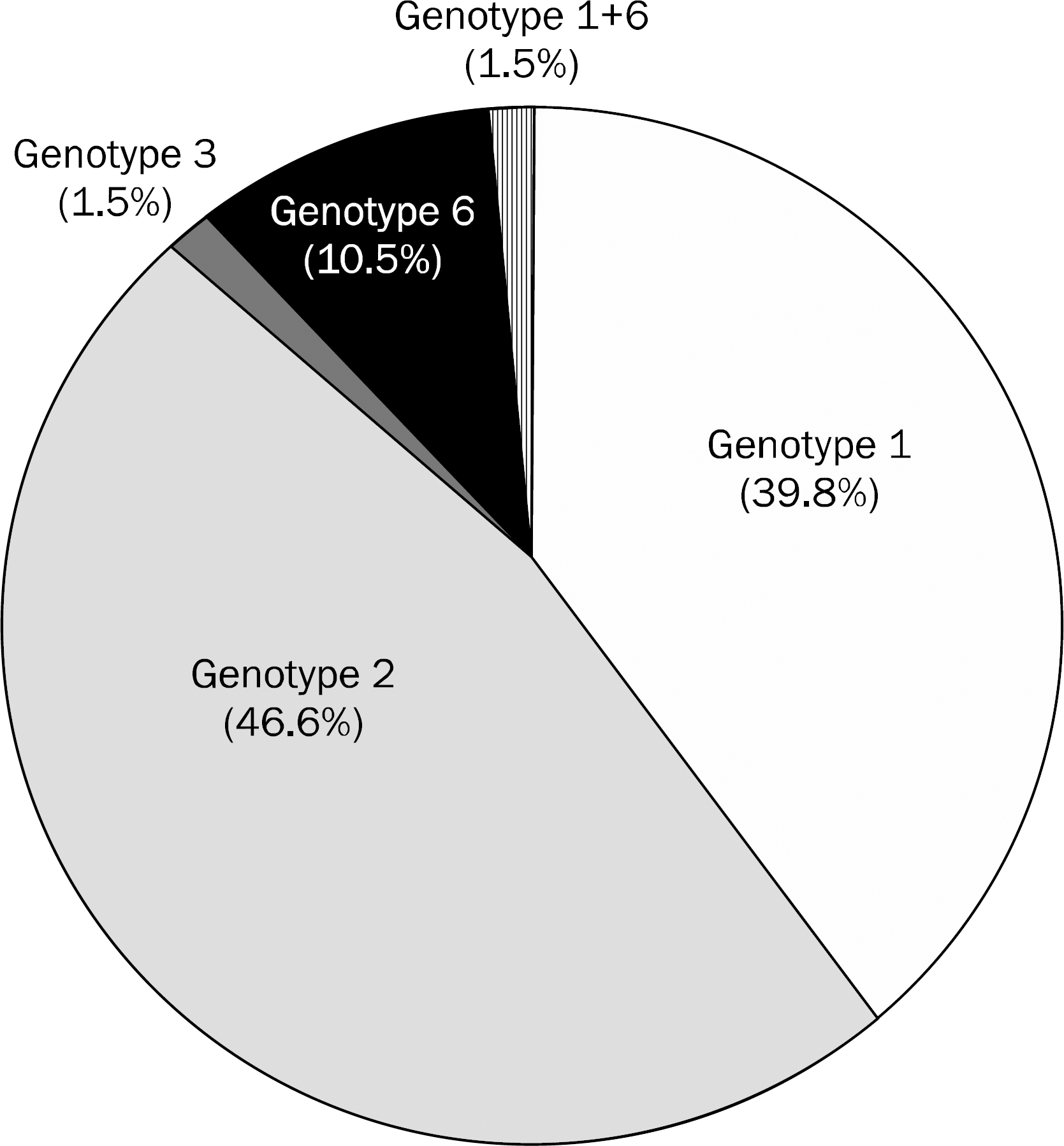
 XML Download
XML Download