Abstract
Background/Aims
Azathioprine (AZA) has been widely used in the therapy of inflammatory bowel disease (IBD) and autoimmune hepatitis (AIH). However, studies evaluating the adverse effects of AZA in these two diseases are lacking. The aim of this study was to compare the adverse effects of AZA in Korean IBD and AIH patients.
Methods
Patients with IBD or AIH who were treated with AZA at Keimyung University Dongsan Medical Center (Daegu, Korea) between January 2002 and March 2011 were enrolled. Their medical records were reviewed retrospectively in terms of clinical characteristics and adverse effects of AZA.
Results
A total of 139 IBD patients and 55 AIH patients were finally enrolled. Thirty IBD patients (21.6%) and eight AIH patients (14.5%) experienced adverse effects of AZA. In particular, the prevalence of leukopenia was significantly higher in the IBD group than in the AIH group (p=0.026). T474C mutation was observed in three of 10 patients who were assessed for thiopurine methyltransferase (TPMT) genotype.
Go to : 
References
1. Kim ES, Kim WH. Inflammatory bowel disease in Korea: epi-demiological, genomic, clinical, and therapeutic characteristics. Gut Liver. 2010; 4:1–14.

2. Liberal R, Grant CR, Longhi MS, Mieli-Vergani G, Vergani D. Diagnostic criteria of autoimmune hepatitis. Autoimmun Rev. 2014; 13:435–440.

3. Bajaj JS, Saeian K, Varma RR, et al. Increased rates of early adverse reaction to azathioprine in patients with Crohn's disease compared to autoimmune hepatitis: a tertiary referral center experience. Am J Gastroenterol. 2005; 100:1121–1125.

4. Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut. 2002; 50:485–489.

5. Manns MP, Czaja AJ, Gorham JD, et al. American Association for the Study of Liver Diseases. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010; 51:2193–2213.

6. Connell WR, Kamm MA, Ritchie JK, Lennard-Jones JE. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut. 1993; 34:1081–1085.

7. Chun JY, Kang B, Lee YM, Lee SY, Kim MJ, Choe YH. Adverse events associated with azathioprine treatment in korean pediatric inflammatory bowel disease patients. Pediatr Gastroenterol Hepatol Nutr. 2013; 16:171–177.

8. de Jong DJ, Goullet M, Naber TH. Side effects of azathioprine in patients with Crohn's disease. Eur J Gastroenterol Hepatol. 2004; 16:207–212.

9. Gisbert JP, Niño P, Rodrigo L, Cara C, Guijarro LG. Thiopurine methyltransferase (TPMT) activity and adverse effects of azathioprine in inflammatory bowel disease: long-term follow-up study of 394 patients. Am J Gastroenterol. 2006; 101:2769–2776.

10. Kim JH, Cheon JH, Kim WH. The frequency and the course of the adverse effects of azathioprine/6-mercaptopurine treatment in patients with inflammatory bowel disease. Korean J Gastroenterol. 2008; 51:291–297.
11. Saibeni S, Virgilio T, D'Incà R, et al. The use of thiopurines for the treatment of inflammatory bowel diseases in clinical practice. Dig Liver Dis. 2008; 40:814–820.

12. Bastida G, Nos P, Aguas M, et al. Incidence, risk factors and clinical course of thiopurine-induced liver injury in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2005; 22:775–782.

13. Ben Ari Z, Mehta A, Lennard L, Burroughs AK. Azathioprine-in-duced myelosuppression due to thiopurine methyltransferase deficiency in a patient with autoimmune hepatitis. J Hepatol. 1995; 23:351–354.
15. George J, Present DH, Pou R, Bodian C, Rubin PH. The long-term outcome of ulcerative colitis treated with 6-mercaptopurine. Am J Gastroenterol. 1996; 91:1711–1714.
16. Sandborn WJ, Tremaine WJ, Wolf DC, et al. Lack of effect of intra-venous administration on time to respond to azathioprine for steroid-treated Crohn's disease. North American Azathioprine Study Group. Gastroenterology. 1999; 117:527–535.
17. Present DH, Meltzer SJ, Krumholz MP, Wolke A, Korelitz BI. 6-Mercaptopurine in the management of inflammatory bowel disease: short- and long-term toxicity. Ann Intern Med. 1989; 111:641–649.

18. Kim JH, Cheon JH, Hong SS, et al. Influences of thiopurine methyltransferase genotype and activity on thiopurine-induced leukopenia in Korean patients with inflammatory bowel disease: a retrospective cohort study. J Clin Gastroenterol. 2010; 44:e242–e248.
19. Kim DU, Kim YH, Kim BJ, et al. The efficacy of low dose azathioprine/6-mercaptopurine in patients with inflammatory bowel disease. Hepatogastroenterology. 2009; 56:1395–1402.
20. Jun JB, Cho DY, Kang C, Bae SC. Thiopurine S-methyltransferase polymorphisms and the relationship between the mutant alleles and the adverse effects in systemic lupus erythematosus patients taking azathioprine. Clin Exp Rheumatol. 2005; 23:873–876.
21. Jung YS, Cheon JH, Park JJ, et al. Correlation of genotypes for thiopurine methyltransferase and inosine triphosphate py-rophosphatase with long-term clinical outcomes in Korean patients with inflammatory bowel diseases during treatment with thiopurine drugs. J Hum Genet. 2010; 55:121–123.

22. Venkat Raman G, Sharman VL, Lee HA. Azathioprine and allo-purinol: a potentially dangerous combination. J Intern Med. 1990; 228:69–71.
23. Szumlanski CL, Weinshilboum RM. Sulphasalazine inhibition of thiopurine methyltransferase: possible mechanism for inter-action with 6-mercaptopurine and azathioprine. Br J Clin Pharmacol. 1995; 39:456–459.

24. Korelitz BI. Steroids may prevent leukopenia, interfering with response to IV azathioprine in treatment of Crohn's disease. Gastroenterology. 2000; 118:1281–1282.
25. Dubinsky MC. Azathioprine, 6-mercaptopurine in inflammatory bowel disease: pharmacology, efficacy, and safety. Clin Gastroenterol Hepatol. 2004; 2:731–743.

Go to : 
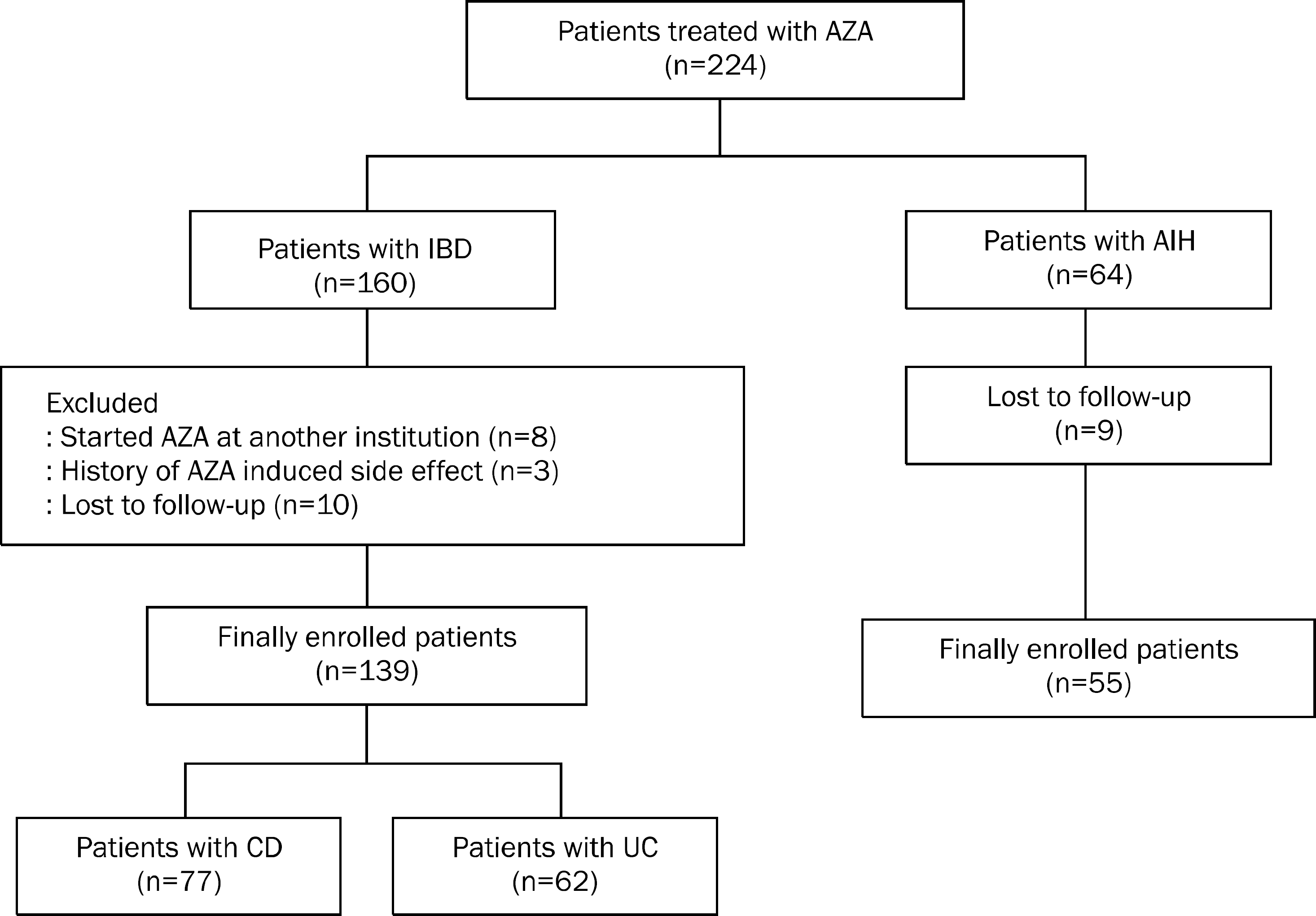 | Fig. 1.Flow diagram of enrolled patients. AZA, azathioprine; IBD, inflammatory bowel disease; AIH, autoimmune hepatitis; 6-MP, 6-mercaptopurine; CD, Crohn's disease; UC, ulcerative colitis. |
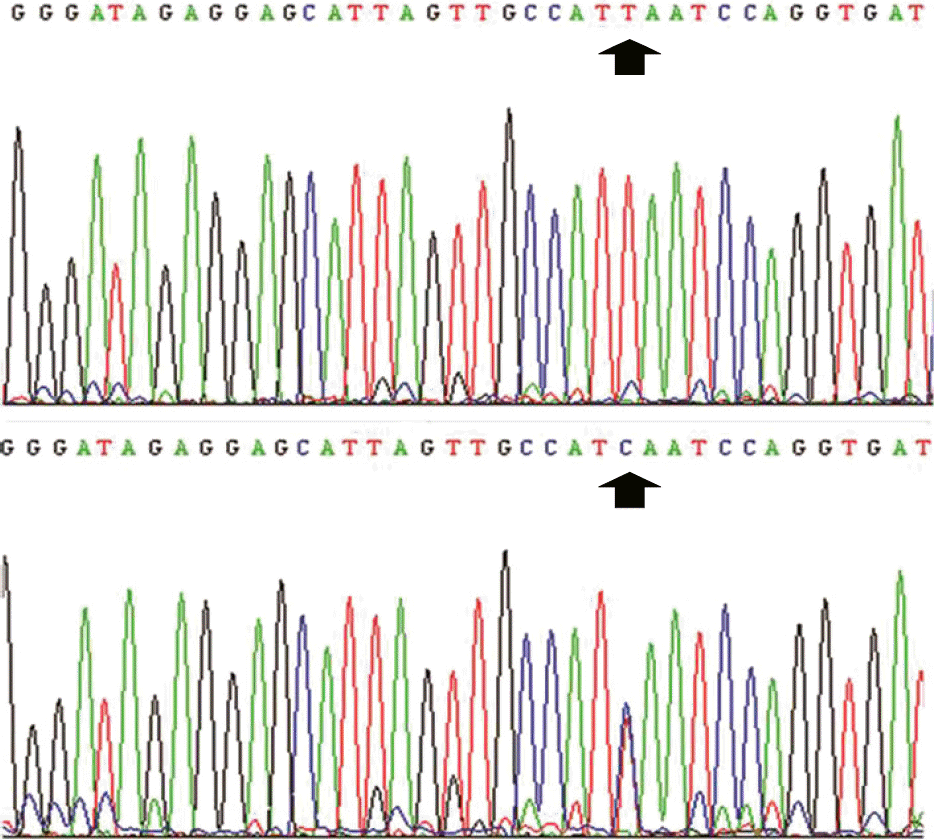 | Fig. 2.Electropherogram showing the alteration of a single nucleotide in the 7th exon. Genotyping of the 7th exon shows that three patients have two different alleles of thymine (T) and cytosine (C). This heterozygous T474C silent mutation was confirmed for TPMT∗1S. G, guanine; A, adenine. |
Table 1.
Characteristics of the Studied Population Treated with Azathioprine
| Characteristic | IBD (n=139 a) | AIH (n=55) | p-value |
|---|---|---|---|
| Age at diagnosed (yr) | 32.23±14.74 | 49.34±15.39 | 0.001 |
| (19–80) | (18–78) | ||
| Sex | 0.001 | ||
| Male | 89 (64.0) | 12 (21.8) | |
| Female | 50 (36.0) | 43 (78.2) | |
| Duration of AZA treatment (mo) | 23.0 (0–93) | 24.57 (0–81) | 0.186 |
| Initial combination therapy with steroid | 77 (55.4) | 55 (100) | 0.001 |
| TPMT genotype b | 1.000 | ||
| TPMT∗1 | 3 (60.0) | 4 (80.0) | |
| TPMT∗1S | 2 (40.0) | 1 (20.0) |
Table 2.
Clinical Details of Patients Who Developed Adverse Effects of Azathioprine
Table 3.
Adverse Effects of Azathioprine by Disease
| Variable | IBD (n=139) | AIH (n=55) | p-value |
|---|---|---|---|
| Total adverse effects a | 30 (21.6) | 8 (14.5) | 0.266 |
| Bone marrow suppression | |||
| Leukopenia | 21 (15.1) | 2 (3.6) | 0.026 |
| Anemia | 6 (4.3) | 2 (3.6) | 1.000 |
| Thrombocytopenia | 2 (1.4) | 3 (5.5) | 0.141 |
| Acute pancreatitis | 3 (2.2) | 0 | 0.564 |
| Nausea/vomiting | 4 (2.9) | 1 (1.8) | 1.000 |
| Skin rash | 1 (0.7) | 1 (1.8) | 0.488 |
Table 4.
Comparisons of Patients with IBD and AIH Who Developed Leukopenia While Taking AZA
Table 5.
Characteristics of Patients Who Were Admitted to Hospital due to Leukopenia




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download