Abstract
Background/Aims
This study was conducted to establish a guideline on the utilizing of feeding pump in patients requiring enteral tube feeding.
Methods
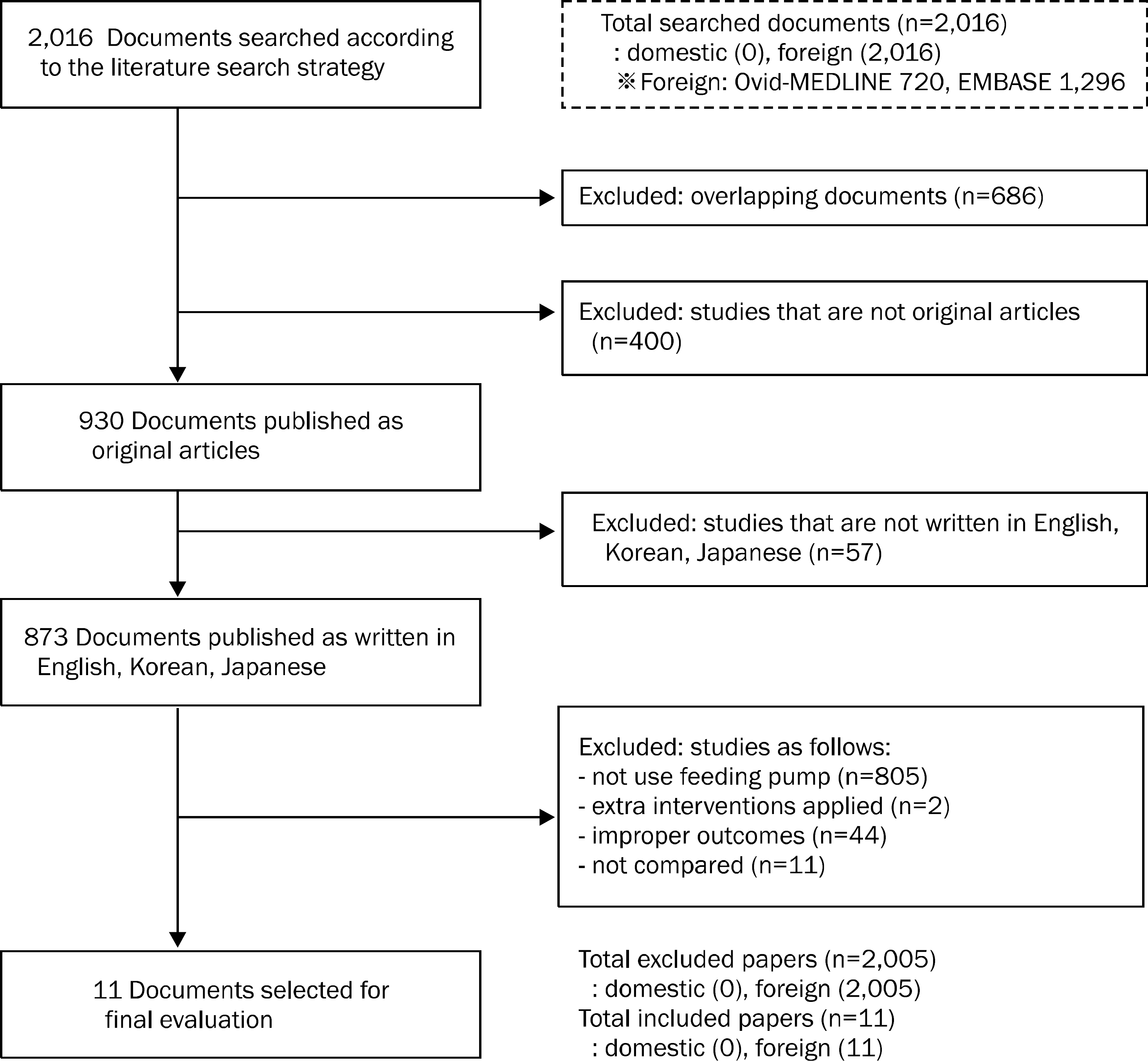
As a first step, textbooks on nutrition and guidelines from regional clinical nutrition societies were analyzed. Afterwards, data on the efficacy, safety, and practicality of feeding pump application were collected and evaluated by systematically reviewing the related literature. As data sources, 8 domestic databases including KoreaMed and global databases such as Ovid-MEDLINE, Ovid-EMBASE, and Cochrane Library were utilized. A total of 2,016 related articles was selected by applying the keyword “(enteral feeding.mp AND pump.mp)”.
Results
Textbooks and guidelines were not able to draw conclusions on the effects of the feeding pump because the injection speed, tube size, and etcetera were different for enteral feeding. Feeding pump assisted enteral tube feeding was an efficient, safe, and practical procedure for reducing maladjustment-related complications of enteral tube feeding, which are obvious obstacles for maintaining nutritional balances in patients requiring tube feeding.
Conclusions
Feeding pump application can be considered an efficient and safe measure that is acceptable in patients on small intestinal tube feeding, critically-ill patients on gastrointestinal tube feeding, premature babies, and critically-ill or severely malnourished children (recommendation grade D).
References
1. Longo DL, Fauci A, Kasper D, et al. Harrison's principles of internal medicine. 18th ed.New York: McGraw-Hill;2011.
2. Kim H, Choi SH, Ham YJ. Nutritional status and indicators of intensive care unit patients on enteral feeding. J Korean Acad Fundam Nurs. 2009; 16:21–29.
3. Kim EM. Feeding pump. J Korean Soc Parenter Enter Nutr. 2010; 3:23–26.
4. Schanler RJ, Garza C, Nichols BL. Fortified mothers’ milk for very low birth weight infants: results of growth and nutrient balance studies. J Pediatr. 1985; 107:437–445.

5. Kim SY. Enteral nutrition in prematurity based on evidence based medicine. J Korean Soc Neonatol. 2007; 14:121–129.
6. Rha MY. Survey of enteral nutrition in Korea. J Korean Soc Parenter Enter Nutr. 2010; 3:9–14.
7. The Korean Dietetic Association. Manual of medical nutrition therapy. 3th ed.Seoul: The Korean Dietetic Association;2008.
8. Bankhead R, Boullata J, Brantley S, et al. A.S.P.E.N. enteral nutrition practice recommendations. JPEN J Parenter Enteral Nutr. 2009; 33:122–167.

9. McClave SA, Martindale RG, Vanek VW, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically Ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2009; 33:277–316.
10. Cano N, Fiaccadori E, Tesinsky P, et al. ESPEN guidelines on enteral nutrition. Clin Nutr. 2006; 25:295–310.
11. Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P. Canadian clinical practice guidelines for nutrition support in me-chanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr. 2003; 27:355–373.

12. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. [Internet]. The Cochrane Collaboration [updated 2011 Mar; cited 2012 Jul 9]. Available from:. http://www.cochrane-handbook.org.
13. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-anal-yses: the PRISMA statement. Ann Intern Med. 2009; 151:264–269.

14. Premji SS, Chessell L. Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams. Cochrane Database Syst Rev. 2011; 11:CD001819.

15. Horn D, Chaboyer W. Gastric feeding in critically ill children: a randomized controlled trial. Am J Crit Care. 2003; 12:461–468.

16. Dollberg S, Kuint J, Mazkereth R, Mimouni FB. Feeding tolerance in preterm infants: randomized trial of bolus and continuous feeding. J Am Coll Nutr. 2000; 19:797–800.

17. Akintorin SM, Kamat M, Pildes RS, et al. A prospective randomized trial of feeding methods in very low birth weight infants. Pediatrics. 1997; 100:e4.

18. Shimoni Z, Averbuch Y, Shir E, et al. The addition of fiber and the use of continuous infusion decrease the incidence of diarrhea in elderly tube-fed patients in medical wards of a general regional hospital: a controlled clinical trial. J Clin Gastroenterol. 2007; 41:901–905.
19. Chen YC, Chou SS, Lin LH, Wu LF. The effect of intermittent nasogastric feeding on preventing aspiration pneumonia in venti-lated critically ill patients. J Nurs Res. 2006; 14:167–180.

20. Horn D, Chaboyer W, Schluter PJ. Gastric residual volumes in critically ill paediatric patients: a comparison of feeding regimens. Aust Crit Care. 2004; 17:98–100. 102-103.

21. Lee JS, Auyeung TW. A comparison of two feeding methods in the alleviation of diarrhoea in older tube-fed patients: a randomised controlled trial. Age Ageing. 2003; 32:388–393.

22. Tamowicz B, Mikstacki A, Grzymislawski M. The influence of the feeding therapy model on pulmonary complications in patients treated under conditions of intensive therapy. Adv Clin Exp Med. 2007; 16:365–373.
23. Ciocon JO, Galindo-Ciocon DJ, Tiessen C, Galindo D. Continuous compared with intermittent tube feeding in the elderly. JPEN J Parenter Enteral Nutr. 1992; 16:525–528.

24. Rojahn A, Lindgren CG. Enteral feeding in infants <1250 g starting within 24 h post-partum. Eur J Pediatr. 2001; 160:629–632.
25. Toce SS, Keenan WJ, Homan SM. Enteral feeding in very-low- birth-weight infants. A comparison of two nasogastric methods. Am J Dis Child. 1987; 141:439–444.
26. SIGN 50: a guideline developer's handbook. [Internet]. Edin-burgh: Scottish Intercollegiate Guidelines Network [updated 2011 Nov; cited 2012 Jul 9]. Available from:. http://www.sign.ac.uk/pdf/sign50.pdf.
27. Health Insurance Review Agency. A study on the construction and management of health technology assessment system. Seoul: Health Insurance Review Agency;2005. p. 227–228.
Table 1.
Ovid-MEDLINE and EMBASE Search Strategy
Table 2.
Levels of Evidence
Table 3.
Grades of Recommendations27
Table 4.
Documents Selected for Evaluation of Feeding Pump
Table 5.
Studies on Safety of the Feeding Pump for Enteral Tube Feeding
Table 6.
Studies on Effectiveness of the Feeding Pump for Enteral Tube Feeding




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download