Abstract
Background/Aims
The diagnostic value of PET-CT, in gastric cancer is well known, but the prognostic value of pretreatment PET-CT has not been adequately evaluated. This study aimed to investigate the preoperative prognostic value of PET-CT in gastric cancer patients.
Methods
A total of 107 patients underwent surgical treatment for gastric cancer from April 2007 to December 2010 at Dong-A University Medical Center after confirming the presence of F-18 fluorodeoxyglucose (FDG) uptake on preoperative PET-CT. Among these patients, the following subjects were excluded: follow-up loss (13), palliative resection (5), neoadjuvant chemotherapy (1), and unrelated death (1). The remaining 87 patients were included in this study and data were collected by retrospectively reviewing the medical records. The median follow-up duration, defined as the period from operation to last imaging study date, was 34.2±14.8 months. FDG uptake values were represented by maximal standardized uptake value (SUVmax). In order to assess the correlation between SUVmax and recurrence, Kaplan-Meier's survival analysis with log-rank test and cox proportional hazard model were performed. Receiver operating characteristic (ROC) curve was employed to determine the optimal cutoff value of SUVmax.
Results
The result of Kaplan-Meier's survival analysis with log-rank test were significantly different between high SUVmax group and low SUVmax group (p=0.035), the cutoff value of which was 5.6. However, in multivariate analysis with cox proportional hazard model, T-staging, N-staging and SUVmax did not show statistical significance (p=0.190, p=0.307, and p=0.436, respectively).
Go to : 
References
1. Reddy NK, Markowitz AB, Abbruzzese JL, Bhutani MS. Knowledge of indications and utilization of EUS: a survey of on-cologists in the United States. J Clin Gastroenterol. 2008; 42:892–896.
2. Fukuya T, Honda H, Kaneko K, et al. Efficacy of helical CT in T-staging of gastric cancer. J Comput Assist Tomogr. 1997; 21:73–81.

3. De Potter T, Flamen P, Van Cutsem E, et al. Whole-body PET with FDG for the diagnosis of recurrent gastric cancer. Eur J Nucl Med Mol Imaging. 2002; 29:525–529.

4. Stahl A, Ott K, Weber WA, et al. FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging. 2003; 30:288–295.

5. Warburg O. Origin of cancer cells. Oncologia. 1956; 9:75–83.
6. Kato H, Miyazaki T, Nakajima M, et al. The incremental effect of positron emission tomography on diagnostic accuracy in the initial staging of esophageal carcinoma. Cancer. 2005; 103:148–156.

7. Kneist W, Schreckenberger M, Bartenstein P, Grünwald F, Oberholzer K, Junginger T. Positron emission tomography for staging esophageal cancer: does it lead to a different therapeutic approach? World J Surg. 2003; 27:1105–1112.

8. Kantorová I, Lipská L, Bêlohlávek O, Visokai V, Trubaĉ M, Schneiderová M. Routine (18)F-FDG PET preoperative staging of colorectal cancer: comparison with conventional staging and its impact on treatment decision making. J Nucl Med. 2003; 44:1784–1788.
9. Chen J, Cheong JH, Yun MJ, et al. Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer. 2005; 103:2383–2390.

10. Kim SK, Kang KW, Lee JS, et al. Assessment of lymph node metastases using 18F-FDG PET in patients with advanced gastric cancer. Eur J Nucl Med Mol Imaging. 2006; 33:148–155.

11. Um SW, Kim H, Koh WJ, et al. Prognostic value of 18F-FDG uptake on positron emission tomography in patients with pathologic stage I non-small cell lung cancer. J Thorac Oncol. 2009; 4:1331–1336.

12. Mukai K, Ishida Y, Okajima K, Isozaki H, Morimoto T, Nishiyama S. Usefulness of preoperative FDG-PET for detection of gastric cancer. Gastric Cancer. 2006; 9:192–196.

13. Yoshioka T, Yamaguchi K, Kubota K, et al. Evaluation of 18F-FDG PET in patients with advanced, metastatic, or recurrent gastric cancer. J Nucl Med. 2003; 44:690–699.
Go to : 
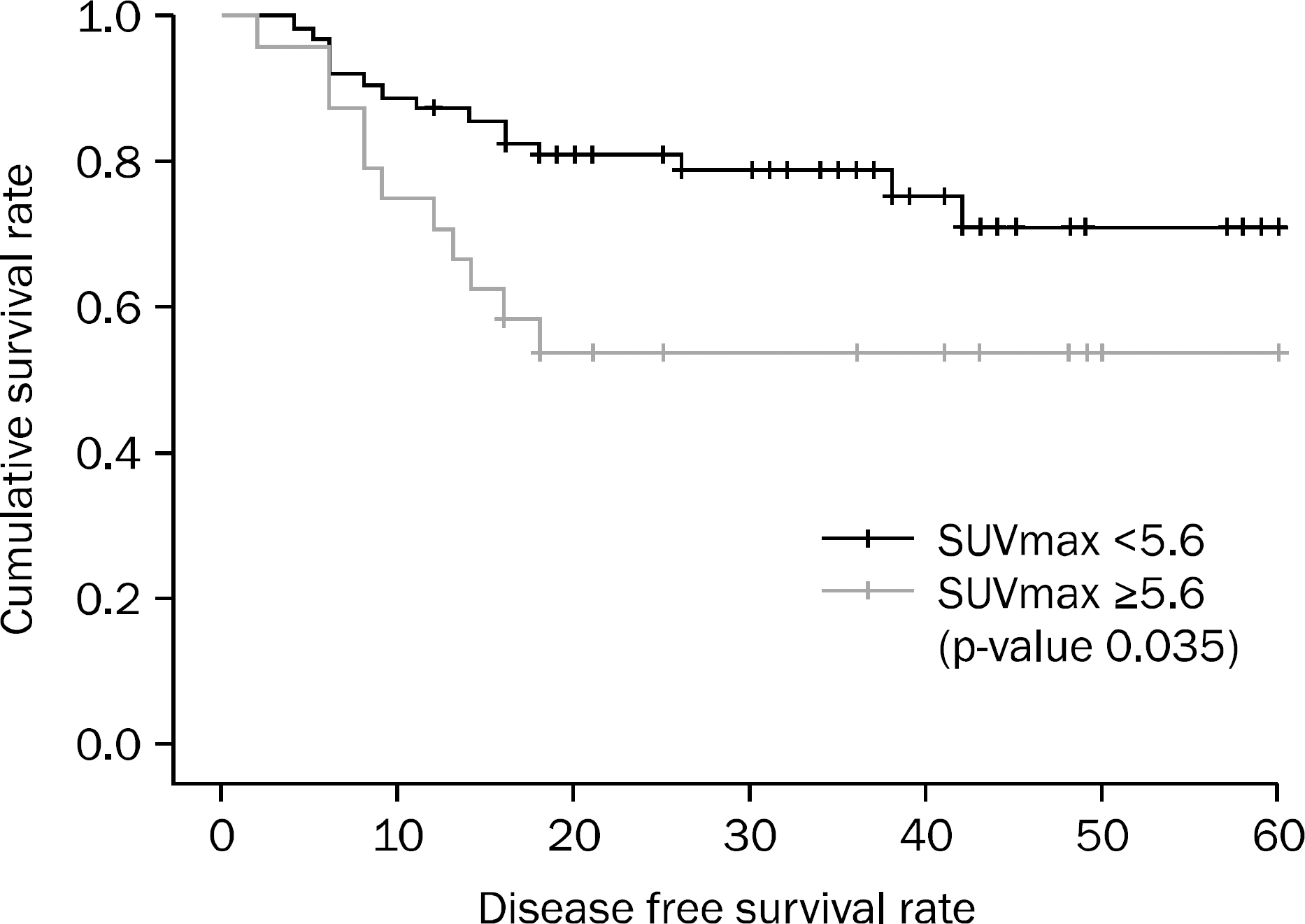 | Fig. 1.Cumulative survival according to maximal standardized uptake value (SUVmax). Cumulative survival showed significant difference between high and low SUVmax group (p=0.035), separated by a SUVmax cutoff value of 5.6 (Kaplan Meier's survival analysis with log rank test). |
Table 1.
Case Characteristics (n=87)
Table 2.
Characteristics of Resected Gastric Cancer Patients
Values are presented as mean±SD or n only. RSG, radical subtotal gastrectomy; RTG, radical total gastrectomy; B-I, Billroth I proceure; B-II, Billroth II procedure; R-Y, Roux-en-Y an-astomosis; WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated; SR, signet-ring cell carcinoma.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download