Abstract
Background/Aims
The incidence of colorectal cancer has been increasing every year in Korea. Irinotecan- or oxaliplatin-based regimens including biologic agents are known to be effective in patients with advanced colorectal cancer. But in practice, FOLFOX (combination of oxaliplatin, 5-fluorouracil, and leucovorin) or FOLFIRI (combination of irinotecan, 5-fluorouracil, and leucovorin) regimens without biologic agents are more commonly used in Korea due to of the high costs of biologic agents. The aim of this study was to evaluate the efficacy and toxicity of FOLFIRI following FOLFOX4 in patients with advanced colorectal cancer.
Methods
A total of 54 patients with advanced colorectal cancer who were treated between May 2005 and May 2013 with FOLFOX4 as first-line chemotherapy and with FOLFIRI as second-line chemotherapy at Kosin University Gospel Hospital (Busan, Korea) were reviewed retrospectively.
Results
A total of 54 patients received second-line FOLFIRI chemotherapy. Five patients (9.3%) had a partial response, 29 patients (53.7%) had a stable disease. The median overall survival was 8.90 months and the median time to progression was 4.33 months. Toxicities were tolerable.
Conclusions
In a Korean population, FOLFIRI as second-line chemotherapy is effective and well tolerated in patients with advanced colorectal cancer after failure of FOLFOX4. Although the efficacy of FOLFIRI in this study was lower than that of second-line FOLFIRI with biologic agents, these results can help in the formulation of a treatment strategy for financially troubled patients.
Go to : 
References
1. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013; 45:1–14.

2. Casado-Saenz E, Feliu J, Gomez-España MA, Sanchez-Gastaldo A, Garcia-Carbonero R. SEOM clinical guidelines for the treatment of advanced colorectal cancer 2013. Clin Transl Oncol. 2013; 15:996–1003.

3. de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000; 18:2938–2947.

4. Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004; 22:229–237.

5. Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000; 355:1041–1047.

6. Rothenberg ML, Oza AM, Bigelow RH, et al. Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: interim results of a phase III trial. J Clin Oncol. 2003; 21:2059–2069.

7. NCCN clinical practice guidelines in oncology. Colon cancer ver2. 2014. [Internet]. Washington (PA): National Comprehensive Cancer Network;2014. [updated 2013 Nov 6; cited 2013 Nov 26]. Available from:. http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
8. Colucci G, Gebbia V, Paoletti G, et al. Gruppo Oncologico Dell'Italia Meridionale. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell'Italia Meridionale. J Clin Oncol. 2005; 23:4866–4875.

9. Hochster HS, Hart LL, Ramanathan RK, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol. 2008; 26:3523–3529.

10. Kawato Y, Aonuma M, Hirota Y, Kuga H, Sato K. Intracellular roles of SN-38, a metabolite of the camptothecin derivative CPT-11, in the antitumor effect of CPT-11. Cancer Res. 1991; 51:4187–4191.
11. Conti JA, Kemeny NE, Saltz LB, et al. Irinotecan is an active agent in untreated patients with metastatic colorectal cancer. J Clin Oncol. 1996; 14:709–715.

12. Pitot HC, Wender DB, O'Connell MJ, et al. Phase II trial of irinotecan in patients with metastatic colorectal carcinoma. J Clin Oncol. 1997; 15:2910–2919.

13. Vanhoefer U, Harstrick A, Achterrath W, Cao S, Seeber S, Rustum YM. Irinotecan in the treatment of colorectal cancer: clinical overview. J Clin Oncol. 2001; 19:1501–1518.
14. Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000; 343:905–914.
15. Goldberg RM, Gill S. Recent phase III trials of fluorouracil, irinotecan, and oxaliplatin as chemotherapy for metastatic colorectal cancer. Cancer Chemother Pharmacol. 2004; 54(Suppl 1):S57–S64.

16. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal fluoropyrimidine and oxaliplatin. Cancer Chemother Pharmacol. 2011; 67:147–152.
17. Kabbinavar FF, Schulz J, McCleod M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005; 23:3697–3705.

18. Beretta GD, Petrelli F, Stinco S, et al. FOLFIRI + bevacizumab as second-line therapy for metastatic colorectal cancer pretreated with oxaliplatin: a pooled analysis of published trials. Med Oncol. 2013; 30:486.

19. Vale CL, Tierney JF, Fisher D, et al. Does anti-EGFR therapy improve outcome in advanced colorectal cancer? A systematic review and metaanalysis. Cancer Treat Rev. 2012; 38:618–625.

20. Lee EK, Revil C, Ngoh CA, et al. Clinical and cost effectiveness of bevacizumab + FOLFIRI combination versus FOLFIRI alone as first-line treatment of metastatic colorectal cancer in South Korea. Clin Ther. 2012; 34:1408–1419.

21. André T, Louvet C, Maindrault-Goebel F, et al. CPT-11 (irinotecan) addition to bimonthly, high-dose leucovorin and bolus and continuous-infusion 5-fluorouracil (FOLFIRI) for pretreated metastatic colorectal cancer. GERCOR. Eur J Cancer. 1999; 35:1343–1347.
22. Bao HY, Fang WJ, Zhang XC, et al. Phase II study of FOLFIRI regimen in patients with advanced colorectal cancer refractory to fluoropyrimidine and oxaliplatin. Cancer Chemother Pharmacol. 2011. 67:147–152.

23. Mabro M, Artru P, André T, et al. A phase II study of FOLFIRI-3 (double infusion of irinotecan combined with LV5FU) after FOLFOX in advanced colorectal cancer patients. Br J Cancer. 2006; 94:1287–1292.

24. Zhang W, Zhao ZY, Wu Q, et al. Multicenter phase II study of modified FOLFIRI regimen in the advanced colorectal cancer patient refractory to fluoropyrimidine and oxaliplatin. Zhonghua Zhong Liu Za Zhi. 2006; 28:788–790.
25. Jeon EK, Hong SH, Kim TH, et al. Modified FOLFIRI as second-line chemotherapy after failure of modified FOLFOX-4 in advanced gastric cancer. Cancer Res Treat. 2011; 43:148–153.

Go to : 
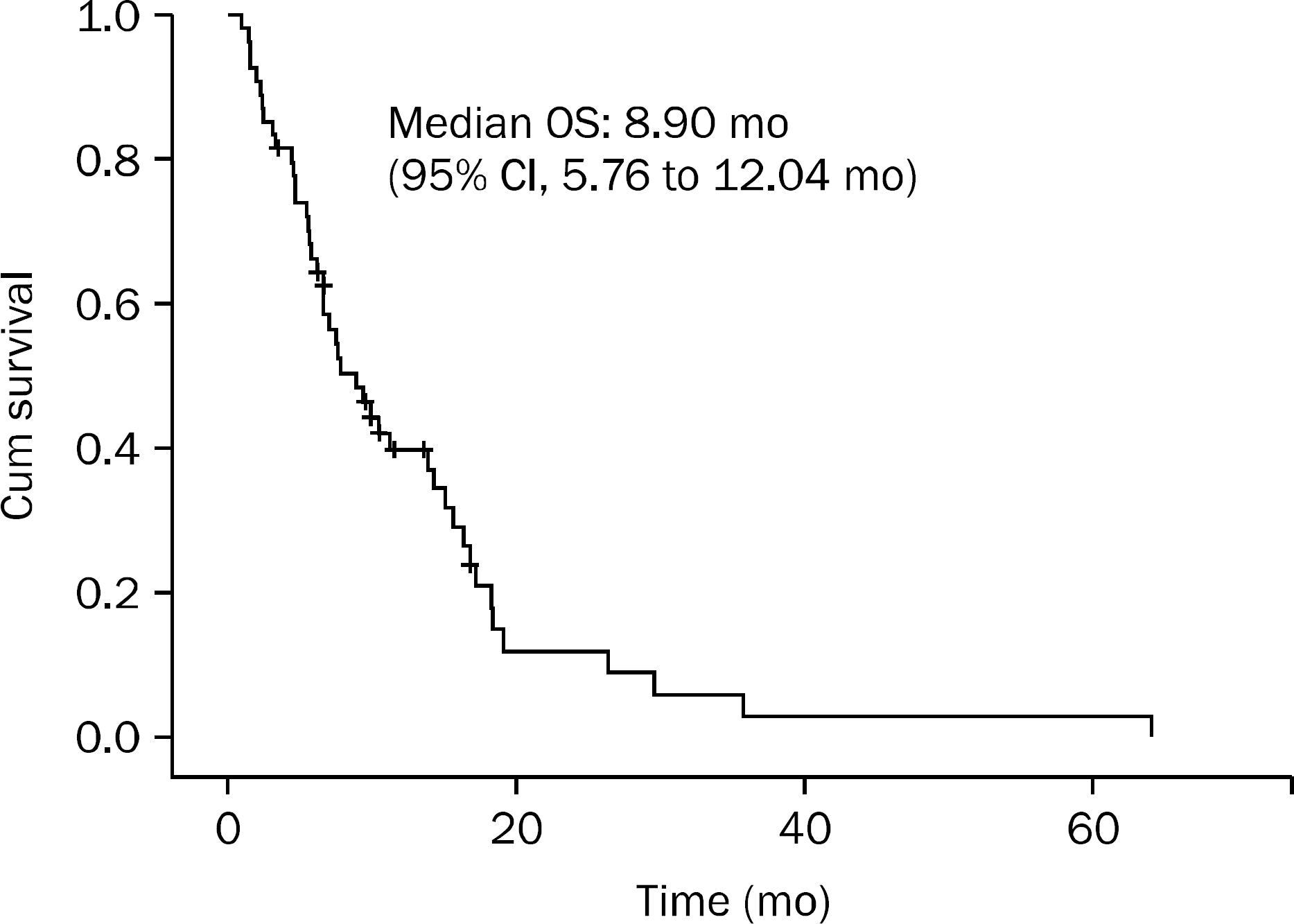 | Fig. 1.Overall survival (OS) curve of patients with advanced colorectal cancer who treated with FOLFIRI after failed FOLFOX4. FOLFIRI, combination of irinotecan, 5-fluorouracil, and leucovorin; FOLFOX, combination of oxaliplatin, 5-fluorouracil, and leucovorin. |
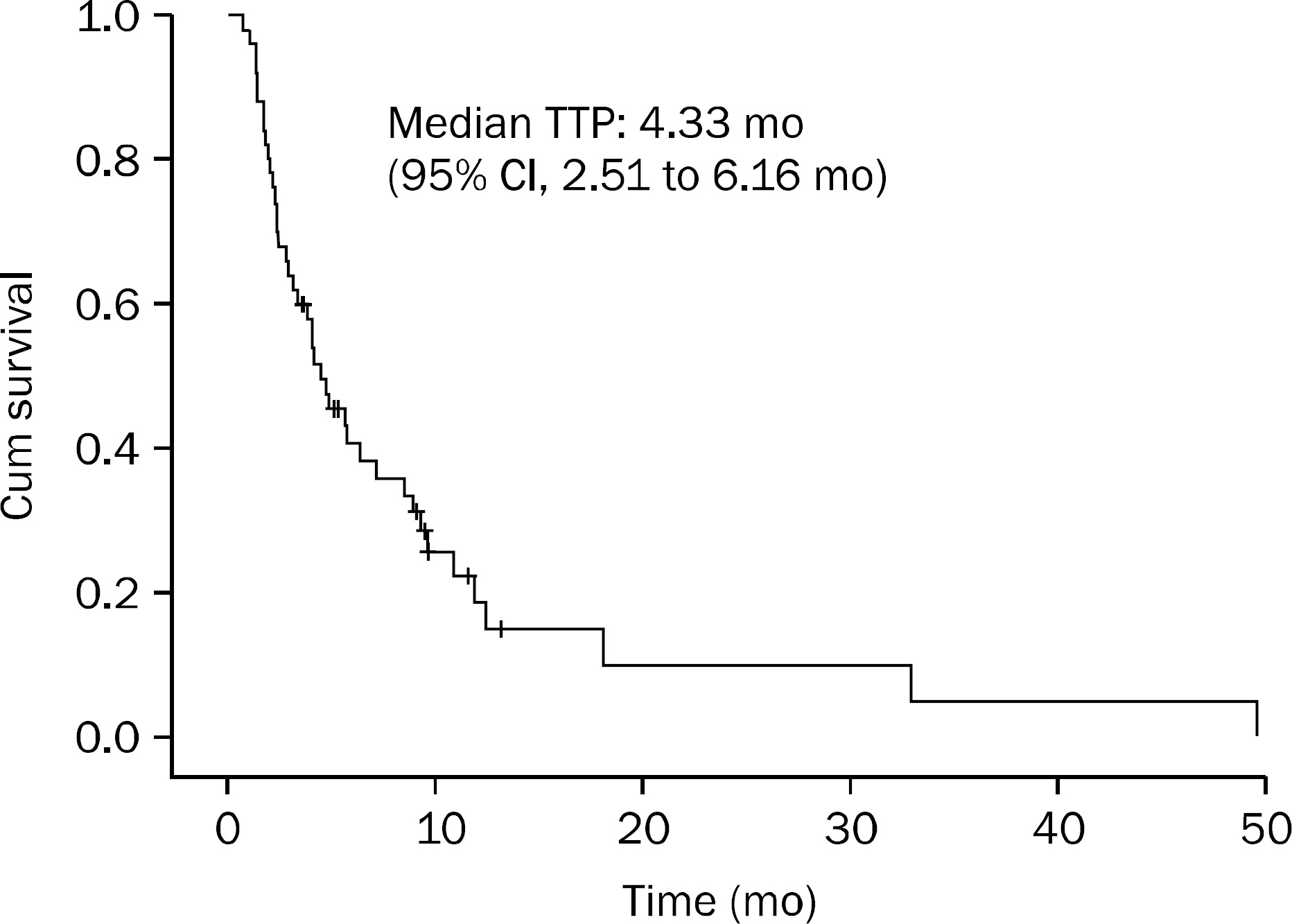 | Fig. 2.Time to progression (TTP) curve of patients with advanced colorectal cancer who treated with FOLFIRI after failed FOLFOX4. FOLFIRI, combination of irinotecan, 5-fluorouracil, and leucovorin; FOLFOX, combination of oxaliplatin, 5-fluorouracil, and leucovorin. |
Table 1.
Patient Characteristics
Table 2.
Treatment Efficacy of FOLFIRI as Second-line Chemotherapy after Failure of FOLFOX4 in Advanced Colorectal Cancer
| Response | Patient |
|---|---|
| Complete response | 0 (0) |
| Partial response | 5 (9.2) |
| Stable disease | 29 (53.7) |
| Progressive disease Not assessable | 13 (24.1) 7 (13.0) |
Table 3.
Toxicity Profiles of FOLFIRI as Second-line Chemotherapy after Failure of FOLFOX4 in Advanced Colorectal Cancer (per Patient)
Table 4.
Comparison with Other Studies Using Second-line FOLFIRI Chemotherapy
| Reference | Patients (n) | Regimen of chemotherapy | RR (%) | OS (median, mo) | TTP (median, mo) | Grade 3–4 netropenia (%) |
|---|---|---|---|---|---|---|
| André et al.21 | 33 | I 180 (D1) for 2 hrs, 5-FU bolus 400 (D1)+infusion 2,400–3,000 (D1-D2) for 46 hrs, LV 400 (D1) for 2 hrs | 5.5 | 9.8 | 4.1 | 15 |
| Tournigand et al.4 | 69 | I 180 (D1) for 2 hrs, 5-FU bolus 400 (D1)+infusion 2,400–3,000 (D1-D2) for 46 hrs, LV 200 (D1) for 2 hrs | 4 | 20.6a | 2.3 | 21 |
| Mabro et al.23 | 65 | I 100 (D1-D2) for 1 hr, 5-FU infusion 2,000 (D1-D2) for 46 hrs, LV 200 (D1) for 2 hrs | 23 | 10.5 | 4.7 | 37 |
| Zhang et al.24 | 80 | I 180 (D1) for 2 hrs, 5-FU bolus 400 (D1)+infusion 2,400 (D1-D2) for 46 hrs, LV 200 (D1) for 2 hrs | 12.5 | NR | 3.2 | 24.1 |
| Bao et al.22 | 57 | I 180 (D1) for 2 hrs, 5-FU bolus 400 (D1)+infusion 2,400 (D1-D2) for 46 hrs, LV 200 (D1) for 2 hrs | 7.5 | 7.8 | 4.8 | 12.9 |
| This study | 54 | I 180 (D1) for 2 hrs, 5-FU bolus 400 (D1)+infusion 600 (D1-D2) for 22 hrs, LV 100 (D1) for 2 hrs | 9.3 | 8.9 | 4.33 | 27.2 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download