Abstract
The prevalence of Helicobacter pylori infection in Korea shows a decreasing trend and has changed to that of developed country, especially for those below 30 years old. However, the primary antibiotic resistance rates are higher than those of developed countries. The reason for the decrease in the efficacy of standard triple therapy is mainly due to the increase in the resistance against clarithromycin. Sequential therapy seems to be more effective than the standard triple therapy, but the intention-to-treat eradication rate of sequential therapy in Korea, which is mostly under 80.0%, is still not satisfactory. Therefore, a promising regimen is needed. Recently, the Japanese health insurance system admitted ‘H. pylori-infected gastritis' as an indication of eradication. Furthermore, the Kyoto Consensus Meeting on H. pylori Gastritis held from January 30th to February 1st, 2014, proposed that ‘all H. pylori positive patients should be offered to receive H. pylori eradication'. This suggests that the concept of eradication has been changed from ‘treatment' to ‘prevention'. Various individualized tailored therapy based on the polymorphism, age and other demographic factors and antibiotic resistance has been attempted to maximize H. pylori eradication therapy. The aim of this article is to review the current epidemiology, H. pylori resistance state, treatment guideline, and to assess the possible future strategy and treatment for H. pylori infection in Korea.
Go to : 
References
1. McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010; 362:1597–1604.
2. NIH Consensus Conference. H. pylori in peptic ulcer disease. NIH Consensus development panel on Helicobacter pylori in peptic ulcer disease. JAMA. 1994; 272:65–69.
3. Graham DY, Lew GM, Klein PD, et al. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. A randomized, controlled study. Ann Intern Med. 1992; 116:705–708.
4. Bayerdörffer E, Neubauer A, Rudolph B, et al. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet. 1995; 345:1591–1594.
5. Kim N, Kim JJ, Choe YH, Kim HS, Kim JI, Chung IS. Korean College of Helicobacter and Upper Gastrointestinal Research; Korean Association of Gastroenterology. Diagnosis and treatment guidelines for Helicobacter pylori infection in Korea. Korean J Gastroenterol. 2009; 54:269–278.
6. Lee JY, Kim N, Kim MS, et al. Factors affecting first-line triple therapy of Helicobacter pylori including CYP2C19 genotype and antibiotic resistance. Dig Dis Sci. 2014. DOI: doi:10.1007/s10620–014–3093–7.1–9.
7. World Gastroenterology Organisation global guideline: Helicobacter pylori in developing countries. J Dig Dis. 2011; 12:319–326.
8. Klein PD, Graham DY, Gaillour A, Opekun AR, Smith EO. Water source as risk factor for Helicobacter pylori infection in Peruvian children. Gastrointestinal Physiology Working Group. Lancet. 1991; 337:1503–1506.
9. al-Moagel MA, Evans DG, Abdulghani ME, et al. Prevalence of Helicobacter (formerly Campylobacter) pylori infection in Saudia Arabia, and comparison of those with and without upper gastrointestinal symptoms. Am J Gastroenterol. 1990; 85:944–948.
10. Malaty HM, Evans DG, Evans DJ Jr, Graham DY. Helicobacter pylori in Hispanics: comparison with blacks and whites of similar age and socioeconomic class. Gastroenterology. 1992; 103:813–816.
11. Graham DY, Malaty HM, Evans DG, Evans DJ Jr, Klein PD, Adam E. Epidemiology of Helicobacter pylori in an asymptomatic population in the United States. Effect of age, race, and socioeconomic status. Gastroenterology. 1991; 100:1495–1501.
12. Malaty HM, Graham DY. Importance of childhood socioeconomic status on the current prevalence of Helicobacter pylori infection. Gut. 1994; 35:742–745.
13. Bures J, Kopácová M, Koupil I, et al. European Society for Primary Care Gastroenterology. Epidemiology of Helicobacter pylori infection in the Czech Republic. Helicobacter. 2006; 11:56–65.
14. Shi R, Xu S, Zhang H, et al. Prevalence and risk factors for Helicobacter pylori infection in Chinese populations. Helicobacter. 2008; 13:157–165.
15. Cheng H, Hu F, Zhang L, et al. Prevalence of Helicobacter pylori infection and identification of risk factors in rural and urban Beijing, China. Helicobacter. 2009; 14:128–133.
16. Kim JH, Kim HY, Kim NY, et al. Korea H pylori Study Group, South Korea. Seroepidemiological study of Helicobacter pylori infection in asymptomatic people in South Korea. J Gastroenterol Hepatol. 2001; 16:969–975.
17. Yim JY, Kim N, Choi SH, et al. Seroprevalence of Helicobacter pylori in South Korea. Helicobacter. 2007; 12:333–340.
18. Lim SH, Kwon JW, Kim N, et al. Prevalence and risk factors of Helicobacter pylori infection in Korea: nationwide multicenter study over 13 years. BMC Gastroenterol. 2013; 13:104.

19. Asaka M, Kimura T, Kudo M, et al. Relationship of Helicobacter pylori to serum pepsinogens in an asymptomatic Japanese population. Gastroenterology. 1992; 102:760–766.
20. Shiota S, Murakami K, Fujioka T, Yamaoka Y. Population-based strategies for Helicobacter pylori-associated disease management: a Japanese perspective. Expert Rev Gastroenterol Hepatol. 2010; 4:149–156.
21. Shiota S, Murakawi K, Suzuki R, Fujioka T, Yamaoka Y. Helicobacter pylori infection in Japan. Expert Rev Gastroenterol Hepatol. 2013; 7:35–40.
22. Nishizawa T, Suzuki H, Suzuki M, Takahashi M, Hibi T. Proton pump inhibitor-amoxicillin-clarithromycin versus proton pump inhibitor-amoxicillin-metronidazole as first-line Helicobacter pylori eradication therapy. J Clin Biochem Nutr. 2012; 51:114–116.
23. Oh HS, Lee DH, Seo JY, et al. Ten-day sequential therapy is more effective than proton pump inhibitor-based therapy in Korea: a prospective, randomized study. J Gastroenterol Hepatol. 2012; 27:504–509.

24. Basu PP, Rayapudi K, Pacana T, Shah NJ, Krishnaswamy N, Flynn M. A randomized study comparing levofloxacin, omeprazole, nitazoxanide, and doxycycline versus triple therapy for the eradication of Helicobacter pylori. Am J Gastroenterol. 2011; 106:1970–1975.
25. Perri F, Villani MR, Festa V, Quitadamo M, Andriulli A. Predictors of failure of Helicobacter pylori eradication with the standard ‘Maastricht triple therapy’. Aliment Pharmacol Ther. 2001; 15:1023–1029.
26. Bigard MA, Delchier JC, Riachi G, Thibault P, Barthelemy P. One-week triple therapy using omeprazole, amoxycillin and clarithromycin for the eradication of Helicobacter pylori in patients with non-ulcer dyspepsia: influence of dosage of omeprazole and clarithromycin. Aliment Pharmacol Ther. 1998; 12:383–388.
27. De Francesco V, Giorgio F, Hassan C, et al. Worldwide H. pylori antibiotic resistance: a systematic review. J Gastrointestin Liver Dis. 2010; 19:409–414.
28. Kobayashi I, Murakami K, Kato M, et al. Changing antimicrobial susceptibility epidemiology of Helicobacter pylori strains in Japan between 2002 and 2005. J Clin Microbiol. 2007; 45:4006–4010.
29. Duck WM, Sobel J, Pruckler JM, et al. Antimicrobial resistance incidence and risk factors among Helicobacter pylori-infected persons, United States. Emerg Infect Dis. 2004; 10:1088–1094.
30. Megraud F, Coenen S, Versporten A, et al. Study Group participants. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013; 62:34–42.
31. Rafeey M, Ghotaslou R, Nikvash S, Hafez AA. Primary resistance in Helicobacter pylori isolated in children from Iran. J Infect Chemother. 2007; 13:291–295.
32. Datta S, Chattopadhyay S, Patra R, et al. Most Helicobacter pylori strains of Kolkata in India are resistant to metronidazole but susceptible to other drugs commonly used for eradication and ulcer therapy. Aliment Pharmacol Ther. 2005; 22:51–57.
33. Sherif M, Mohran Z, Fathy H, Rockabrand DM, Rozmajzl PJ, Frenck RW. Universal high-level primary metronidazole resistance in Helicobacter pylori isolated from children in Egypt. J Clin Microbiol. 2004; 42:4832–4834.
34. Miyachi H, Miki I, Aoyama N, et al. Primary levofloxacin resistance and gyrA/B mutations among Helicobacter pylori in Japan. Helicobacter. 2006; 11:243–249.
35. Toracchio S, Marzio L. Primary and secondary antibiotic resistance of Helicobacter pylori strains isolated in central Italy during the years 1998–2002. Dig Liver Dis. 2003; 35:541–545.
36. Boyanova L. Prevalence of multidrug-resistant Helicobacter pylori in Bulgaria. J Med Microbiol. 2009; 58:930–935.
37. Asrat D, Kassa E, Mengistu Y, Nilsson I, Wadström T. Antimicrobial susceptibility pattern of Helicobacter pylori strains isolated from adult dyspeptic patients in Tikur Anbassa University Hospital, Addis Ababa, Ethiopia. Ethiop Med J. 2004; 42:79–85.
38. Nahar S, Mukhopadhyay AK, Khan R, et al. Antimicrobial susceptibility of Helicobacter pylori strains isolated in Bangladesh. J Clin Microbiol. 2004; 42:4856–4858.
39. Boyanova L, Mitov I. Geographic map and evolution of primary Helicobacter pylori resistance to antibacterial agents. Expert Rev Anti Infect Ther. 2010; 8:59–70.
40. Vakil N, Vaira D. Treatment for H. pylori infection: new challenges with antimicrobial resistance. J Clin Gastroenterol. 2013; 47:383–388.
41. Su P, Li Y, Li H, et al. Antibiotic resistance of Helicobacter pylori isolated in the Southeast Coastal Region of China. Helicobacter. 2013; 18:274–279.
42. Kim JM, Kim JS, Jung HC, Kim N, Kim YJ, Song IS. Distribution of antibiotic MICs for Helicobacter pylori strains over a 16-year period in patients from Seoul, South Korea. Antimicrob Agents Chemother. 2004; 48:4843–4847.
43. Lee JW, Kim N, Kim JM, et al. Prevalence of primary and secondary antimicrobial resistance of Helicobacter pylori in Korea from 2003 through 2012. Helicobacter. 2013; 18:206–214.
44. Kim JJ, Reddy R, Lee M, et al. Analysis of metronidazole, clarithromycin and tetracycline resistance of Helicobacter pylori isolates from Korea. J Antimicrob Chemother. 2001; 47:459–461.
45. Kim JY, Kim NY, Kim SJ, et al. Regional difference of antibiotic resistance of Helicobacter pylori strains in Korea. Korean J Gastroenterol. 2011; 57:221–229.
46. Kim JM. Antibiotic resistance of Helicobacter pylori isolated from Korean patients. Korean J Gastroenterol. 2006; 47:337–349.
47. Asaka M, Kato M, Takahashi S, et al. Japanese Society for Helicobacter Research. Guidelines for the management of Helicobacter pylori infection in Japan:2009 revised edition. Helicobacter. 2010; 15:1–20.
48. Kim SG, Jung HK, Lee HL, et al. Korean College of Helicobacter and Upper Gastrointestinal Research. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. Korean J Gastroenterol. 2013; 62:3–26.
49. Malfertheiner P, Megraud F, O'Morain CA, et al. European Helicobacter Study Group. Management of Helicobacter pylori infection–the Maastricht IV/Florence Consensus Report. Gut. 2012; 61:646–664.
50. Chey WD, Wong BC. Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007; 102:1808–1825.
51. Fock KM, Katelaris P, Sugano K, et al. Second Asia-Pacific Conference. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009; 24:1587–1600.
52. Chinese Society of Gastroenterology, Chinese Study Group on Helicobacter pylori. Liu WZ, et al. Fourth Chinese National Consensus Report on the management of Helicobacter pylori infection. J Dig Dis. 2013; 14:211–221.
53. Fukase K, Kato M, Kikuchi S, et al. Japan Gast Study Group. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008; 372:392–397.
54. Gisbert JP, Khorrami S, Carballo F, Calvet X, Gené E, Dominguez-Muñoz JE. H. pylori eradication therapy vs. anti-secretory non-eradication therapy (with or without long-term maintenance antisecretory therapy) for the prevention of recurrent bleeding from peptic ulcer. Cochrane Database Syst Rev. 2004; (2):CD004062.

55. Wong BC, Lam SK, Wong WM, et al. China Gastric Cancer Study Group. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004; 291:187–194.
56. Ito M, Takata S, Tatsugami M, et al. Clinical prevention of gastric cancer by Helicobacter pylori eradication therapy: a systematic review. J Gastroenterol. 2009; 44:365–371.
57. Kodama M, Murakami K, Okimoto T, et al. Helicobacter pylori eradication improves gastric atrophy and intestinal metaplasia in long-term observation. Digestion. 2012; 85:126–130.
58. Kodama M, Murakami K, Okimoto T, et al. Ten-year prospective follow-up of histological changes at five points on the gastric mucosa as recommended by the updated Sydney system after Helicobacter pylori eradication. J Gastroenterol. 2012; 47:394–403.
59. de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012; 13:607–615.

60. Choi IJ. Current evidence of effects of Helicobacter pylori eradication on prevention of gastric cancer. Korean J Intern Med. 2013; 28:525–537.
61. Lee YC, Chen TH, Chiu HM, et al. The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention. Gut. 2013; 62:676–682.
62. Ma JL, Zhang L, Brown LM, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst. 2012; 104:488–492.
63. Asaka M, Kato M, Graham DY. Strategy for eliminating gastric cancer in Japan. Helicobacter. 2010; 15:486–490.

64. Asaka M. A new approach for elimination of gastric cancer deaths in Japan. Int J Cancer. 2013; 132:1272–1276.

65. Wu CY, Kuo KN, Wu MS, Chen YJ, Wang CB, Lin JT. Early Helicobacter pylori eradication decreases risk of gastric cancer in patients with peptic ulcer disease. Gastroenterology. 2009; 137:1641–1648. e1–2.
66. Asaka M, Kato M, Graham DY. Prevention of gastric cancer by Helicobacter pylori eradication. Intern Med. 2010; 49:633–636.
67. Lam SK, Talley NJ. Report of the 1997 Asia Pacific Consensus Conference on the management of Helicobacter pylori infection. J Gastroenterol Hepatol. 1998; 13:1–12.
68. European Helicobacter pylori Study Group. Current European concepts in the management of Helicobacter pylori infection. The Maastricht Consensus Report. Gut. 1997; 41:8–13.
69. Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010; 59:1143–1153.
70. Zullo A, Rinaldi V, Winn S, et al. A new highly effective short-term therapy schedule for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2000; 14:715–718.
71. Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008; 148:923–931.
72. Gatta L, Vakil N, Leandro G, Di Mario F, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and metaanalysis of randomized controlled trials in adults and children. Am J Gastroenterol. 2009; 104:3069–3079.
73. Tong JL, Ran ZH, Shen J, Xiao SD. Sequential therapy vs. standard triple therapies for Helicobacter pylori infection: a metaanalysis. J Clin Pharm Ther. 2009; 34:41–53.
74. Urgesi R, Pelecca G, Cianci R, et al. Helicobacter pylori infection: is sequential therapy superior to standard triple therapy? A single-centre Italian study in treatmentnaive and non-treatmentnaive patients. Can J Gastroenterol. 2011; 25:315–318.
75. Paoluzi OA, Visconti E, Andrei F, et al. Ten and eight-day sequential therapy in comparison to standard triple therapy for eradicating Helicobacter pylori infection: a randomized controlled study on efficacy and tolerability. J Clin Gastroenterol. 2010; 44:261–266.
76. Romano M, Cuomo A, Gravina AG, et al. Empirical levofloxacin-containing versus clarithromycin-containing sequential therapy for Helicobacter pylori eradication: a randomised trial. Gut. 2010; 59:1465–1470.
77. Zullo A, Scaccianoce G, De Francesco V, et al. Concomitant, sequential, and hybrid therapy for H. pylori eradication: a pilot study. Clin Res Hepatol Gastroenterol. 2013; 37:647–650.
78. Molina-Infante J, Perez-Gallardo B, Fernandez-Bermejo M, et al. Clinical trial: clarithromycin vs. levofloxacin in first-line triple and sequential regimens for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2010; 31:1077–1084.
79. McNicholl AG, Marin AC, Molina-Infante J, et al. Participant Centres. Randomised clinical trial comparing sequential and concomitant therapies for Helicobacter pylori eradication in routine clinical practice. Gut. 2014; 63:244–249.
80. Gatta L, Vakil N, Vaira D, Scarpignato C. Global eradication rates for Helicobacter pylori infection: systematic review and metaanalysis of sequential therapy. BMJ. 2013; 347:f4587.
81. Kim JS, Kim BW, Ham JH, et al. Sequential therapy for Helicobacter pylori infection in Korea: systematic review and metaanalysis. Gut Liver. 2013; 7:546–551.
82. Yoon H, Lee DH, Kim N, et al. Meta-analysis: is sequential therapy superior to standard triple therapy for Helicobacter pylori infection in Asian adults? J Gastroenterol Hepatol. 2013; 28:1801–1809.
83. Vaira D, Zullo A, Vakil N, et al. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a randomized trial. Ann Intern Med. 2007; 146:556–563.
84. Zullo A, Perna F, Hassan C, et al. Primary antibiotic resistance in Helicobacter pylori strains isolated in northern and central Italy. Aliment Pharmacol Ther. 2007; 25:1429–1434.
85. Chung JW, Jung YK, Kim YJ, et al. Ten-day sequential versus triple therapy for Helicobacter pylori eradication: a prospective, open-label, randomized trial. J Gastroenterol Hepatol. 2012; 27:1675–1680.
86. Abadi AT, Taghvaei T, Mobarez AM, Carpenter BM, Merrell DS. Frequency of antibiotic resistance in Helicobacter pylori strains isolated from the northern population of Iran. J Microbiol. 2011; 49:987–993.
87. Thyagarajan SP, Ray P, Das BK, et al. Geographical difference in antimicrobial resistance pattern of Helicobacter pylori clinical isolates from Indian patients: multicentric study. J Gastroenterol Hepatol. 2003; 18:1373–1378.
88. Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol. 2014; 12:177–186.e3.
89. Essa AS, Kramer JR, Graham DY, Treiber G. Meta-analysis: four-drug, three-antibiotic, non-bismuth-containing "concomitant therapy" versus triple therapy for Helicobacter pylori eradication. Helicobacter. 2009; 14:109–118.
90. Fischbach LA, van Zanten S, Dickason J. Meta-analysis: the efficacy, adverse events, and adherence related to first-line anti-Helicobacter pylori quadruple therapies. Aliment Pharmacol Ther. 2004; 20:1071–1082.
91. Gisbert JP, Calvet X. Review article: non-bismuth quadruple (concomitant) therapy for eradication of Helicobater pylori. Aliment Pharmacol Ther. 2011; 34:604–617.
92. Kim SY, Lee SW, Hyun JJ, et al. Comparative study of Helicobacter pylori eradication rates with 5-day quadruple "concomitant" therapy and 7-day standard triple therapy. J Clin Gastroenterol. 2013; 47:21–24.
93. Hsu PI, Wu DC, Wu JY, Graham DY. Modified sequential Helicobacter pylori therapy: proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (hybrid) therapy for the final 7 days. Helicobacter. 2011; 16:139–145.
94. Sardarian H, Fakheri H, Hosseini V, et al. Comparison of hybrid and sequential therapies for Helicobacter pylori eradication in Iran: a prospective randomized trial. Helicobacter. 2013; 18:129–134.
95. Molina-Infante J, Romano M, Fernandez-Bermejo M, et al. Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology. 2013; 145:121–128.e1.
96. Suzuki T, Matsuo K, Ito H, et al. Smoking increases the treatment failure for Helicobacter pylori eradication. Am J Med. 2006; 119:217–224.
97. Sargýn M, Uygur-Bayramicli O, Sargýn H, Orbay E, Yavuzer D, Yayla A. Type 2 diabetes mellitus affects eradication rate of Helicobacter pylori. World J Gastroenterol. 2003; 9:1126–1128.
98. Osawa H, Kawakami M, Fujii M, et al. Helicobacter pylori infection and coronary heart disease in Japanese patients. Cardiology. 2001; 95:14–19.
99. Aydemir S, Boyacioglu S, Gur G, et al. Helicobacter pylori infection in hemodialysis patients: susceptibility to amoxicillin and clarithromycin. World J Gastroenterol. 2005; 11:842–845.
100. Dickson EJ, Stuart RC. Genetics of response to proton pump inhibitor therapy: clinical implications. Am J Pharmacogenomics. 2003; 3:303–315.
101. Chong E, Ensom MH. Pharmacogenetics of the proton pump inhibitors: a systematic review. Pharmacotherapy. 2003; 23:460–471.

102. Padol S, Yuan Y, Thabane M, Padol IT, Hunt RH. The effect of CYP2C19 polymorphisms on H. pylori eradication rate in dual and triple first-line PPI therapies: a metaanalysis. Am J Gastroenterol. 2006; 101:1467–1475.
103. McNicholl AG, Linares PM, Nyssen OP, Calvet X, Gisbert JP. Meta-analysis: esomeprazole or rabeprazole vs. first-gen-eration pump inhibitors in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 2012; 36:414–425.
104. Zhao F, Wang J, Yang Y, et al. Effect of CYP2C19 genetic polymorphisms on the efficacy of proton pump inhibitor-based triple therapy for Helicobacter pylori eradication: a metaanalysis. Helicobacter. 2008; 13:532–541.
105. Tang HL, Li Y, Hu YF, Xie HG, Zhai SD. Effects of CYP2C19 loss-of-function variants on the eradication of H. pylori infection in patients treated with proton pump inhibitor-based triple therapy regimens: a metaanalysis of randomized clinical trials. PLoS One. 2013; 8:e62162.
106. Lee JH, Jung HY, Choi KD, Song HJ, Lee GH, Kim JH. The influence of CYP2C19 polymorphism on eradication of Helicobacter pylori: a prospective randomized study of lansoprazole and rabeprazole. Gut Liver. 2010; 4:201–206.
107. Kang JM, Kim N, Lee DH, et al. Effect of the CYP2C19 polymorphism on the eradication rate of Helicobacter pylori infection by 7-day triple therapy with regular proton pump inhibitor dosage. J Gastroenterol Hepatol. 2008; 23:1287–1291.
108. Houben MH, van de Beek D, Hensen EF, de Craen AJ, Rauws EA, Tytgat GN. A systematic review of Helicobacter pylori eradication therapy–the impact of antimicrobial resistance on eradication rates. Aliment Pharmacol Ther. 1999; 13:1047–1055.
109. Kim N, Kim JM, Kim CH, et al. Institutional difference of antibiotic resistance of Helicobacter pylori strains in Korea. J Clin Gastroenterol. 2006; 40:683–687.
110. Hwang TJ, Kim N, Kim HB, et al. Change in antibiotic resistance of Helicobacter pylori strains and the effect of A2143G point mutation of 23S rRNA on the eradication of H. pylori in a single center of Korea. J Clin Gastroenterol. 2010; 44:536–543.
111. Woo HY, Park DI, Park H, et al. Dual-priming oligonucleotide-based multiplex PCR for the detection of Helicobacter pylori and determination of clarithromycin resistance with gastric biopsy specimens. Helicobacter. 2009; 14:22–28.
112. Gerrits MM, van Vliet AH, Kuipers EJ, Kusters JG. Helicobacter pylori and antimicrobial resistance: molecular mechanisms and clinical implications. Lancet Infect Dis. 2006; 6:699–709.
113. Shin WG. New trend of Helicobacter pylori treatment. Korean J Med. 2013; 85:586–588.
114. Lee HJ, Kim JI, Cheung DY, et al. Eradication of Helicobacter pylori according to 23S ribosomal RNA point mutations associated with clarithromycin resistance. J Infect Dis. 2013; 208:1123–1130.
115. Furuta T, Shirai N, Kodaira M, et al. Pharmacogenomics-based tailored versus standard therapeutic regimen for eradication of H. pylori. Clin Pharmacol Ther. 2007; 81:521–528.
Go to : 
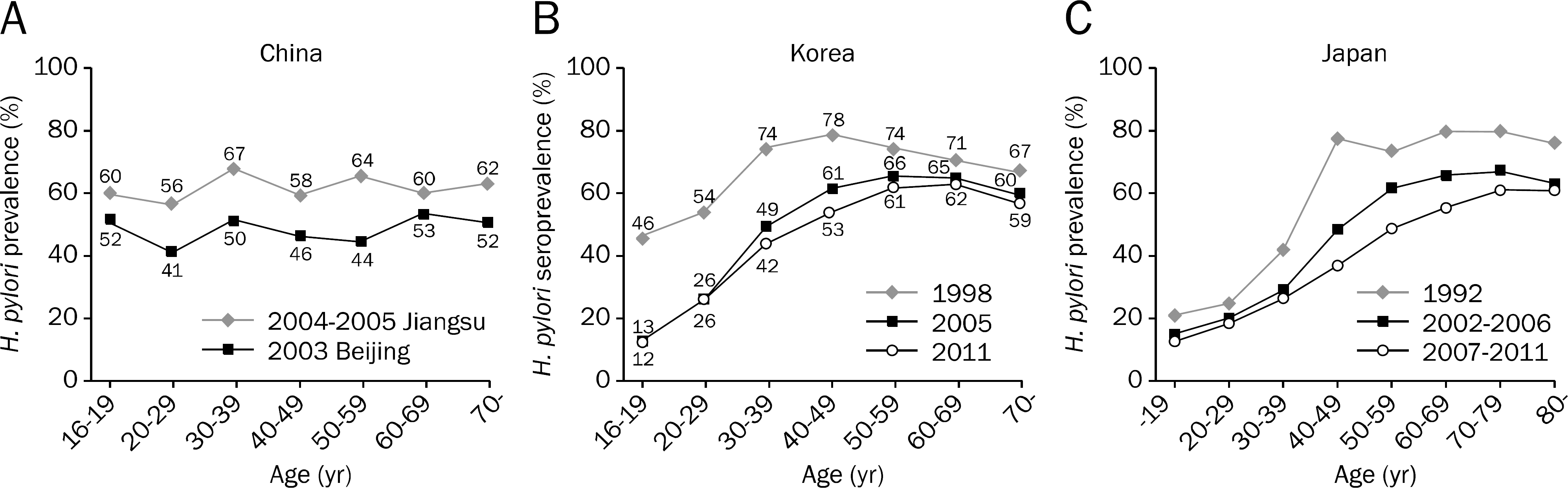 | Fig. 1.Comparison of prevalence rate of Helicobacter pylori infection among China, Korea, and Japan. (A) Prevalence (using urea breath test or serum IgG antibody) by age in 2004–2005 in Jiangsu, China and in 2003 in Beijing, China.14,15 (B) Seroprevalence in asymptomatic subjects without a history of H. pylori eradication in 1998, 2005, and 2011 in Korea.16–18 (C) Prevalence (using urine antibody or serum IgG antibody) of H. pylori in 1992, 2002–2006, and 2007–2011 in Japan.19–21 Reused from the article of Shiota, et al. (Expert Rev Gastroenterol Hepatol 2013;7:35–40)21 with original copyright holder' s permission. |
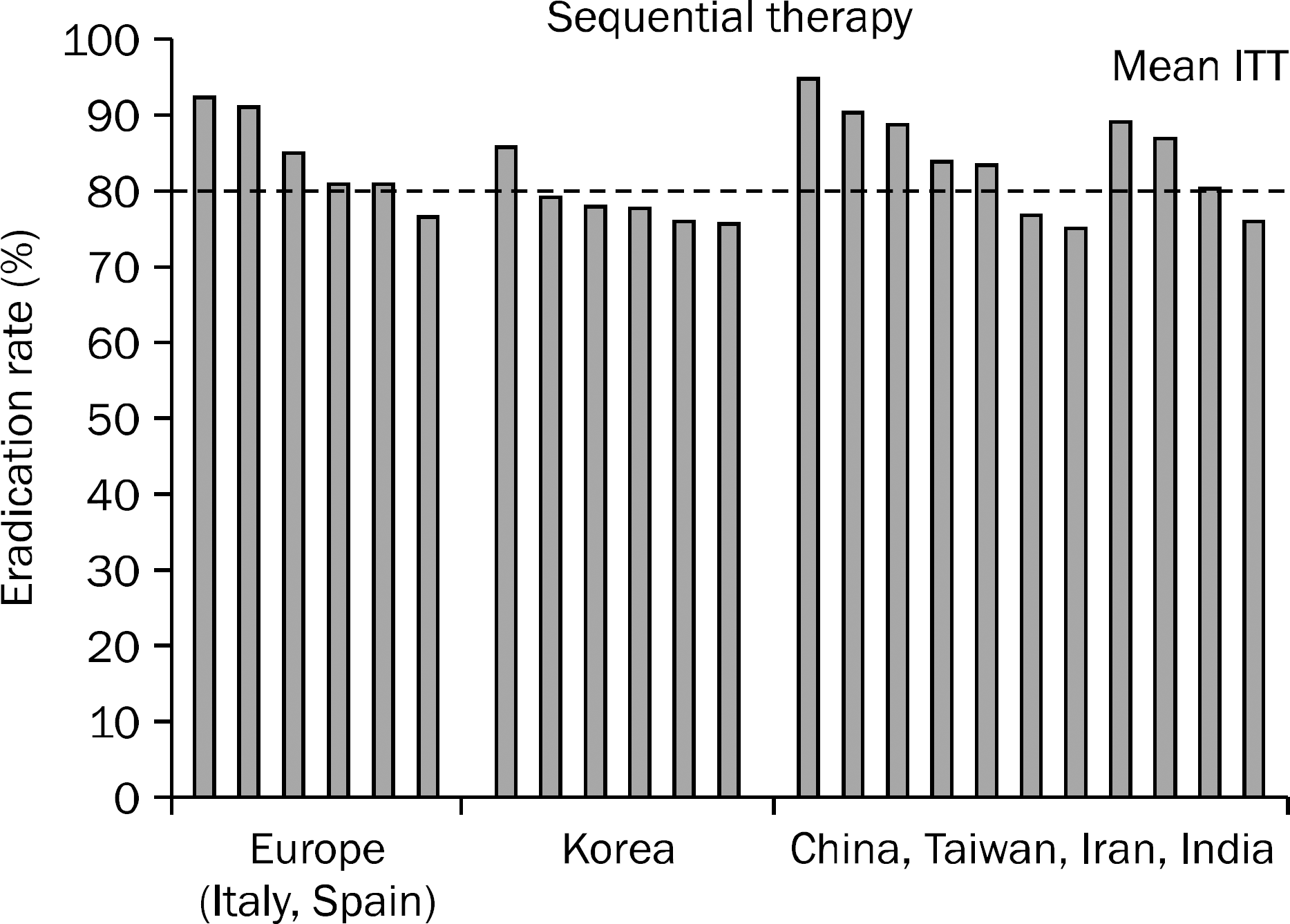 | Fig. 2.The mean intention-to-treat (ITT) eradication rate for sequential therapy reported after 2008 in Europe (Italy,74–79 Spain78,79), Korea,82 and Asia (China, Taiwan, India, and Iran).82
|
Table 1.
Antibiotics Resistance Rates (%) for Helicobacter pylori Infection around the World
Table 2.
Antibiotic Resistance Rates for Helicobacter pylori in Korea
Table 3.
Treatment Indication and Recommended First-line Regimen against Helicobacter pylori Infection by Japanese, Korean, and European Guideline
EMR, endoscopic mucosal resection; EGC, early gastric cancer; MALToma, mucosal associated lymphoid tissue lymphoma; PPI, proton pump inhibitor; Clari-R, clarithromycin resistance; b.i.d., bis in die (twice a day).
Table 4.
Current Recommended Regimens against Helicobacter pylori Infection
Table 5.
Summary of Meta-analysis Investigating the Efficacy of Sequential therapy Compared with Standard Triple Therapy




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download