Abstract
Korea and Japan show the highest incidence of gastric cancer and Helicobacter pylori infection. New 2013 guidelines on H. pylori infection differ between the two countries with regard to the indications for H. pylori eradication, diagnostic methods, and treatment regimens. Indications for eradication in Korean guideline focus on specific diseases such as peptic ulcer disease, low-grade gastric mucosa-associated lymphoid tissue lymphoma, and after resection of early gastric cancer, while Japanese guideline includes all H. pylori-associated gastritis for the prevention of dissemination. With regard to the diagnosis, either noninvasive or invasive method (except for bacterial culture) is recommended in Korea, while two noninvasive tests including serum anti-H. pylori IgG antibody level are preferred in Japan. As for the treatment regimens, second-line treatment (quadruple bismuth-containing regimen) is recommended without first-line triple therapy in areas of high clarithromycin resistance in Korea. However, there is no bismuth-based second-line treatment in Japan, and the Japanese regimen consists of a lower dose of antibiotics for a shorter duration (7 days). Such discrepancies between the two countries are based not only on the differences in the literature search and interpretation, but also on the different approvals granted by the national health insurance system, manufacturing process of the antibiotics, and diagnostic techniques in each country. Collaborations are required to minimize the discrepancies between the two countries based on cost-effectiveness.
Go to : 
References
1. Matsuhisa T, Aftab H. Observation of gastric mucosa in Bangladesh, the country with the lowest incidence of gastric cancer, and Japan, the country with the highest incidence. Helicobacter. 2012; 17:396–401.
2. Nguyen LT, Uchida T, Kuroda A, et al. Evaluation of the anti-East Asian CagA-specific antibody for CagA phenotyping. Clin Vaccine Immunol. 2009; 16:1687–1692.

3. Seo TH, Lee SY, Uchida T, et al. The origin of non-H. pylori-related positive Giemsa staining in human gastric biopsy specimens: a prospective study. Dig Liver Dis. 2011; 43:23–27.
4. Lee SY. Future candidates for indications of Helicobacter pylori eradication: do the indications need to be revised? J Gastroenterol Hepatol. 2012; 27:200–211.
5. Asaka M. A new approach for elimination of gastric cancer deaths in Japan. Int J Cancer. 2013; 132:1272–1276.

6. Lee SY. Gastric adenoma with low-grade dysplasia: two countries, two outcomes. Dig Dis Sci. 2014; 59:235–237.

7. Kim SG, Jung HK, Lee HL, et al. Korean College of Helicobacter and Upper Gastrointestinal Research. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. Korean J Gastroenterol. 2013; 62:3–26.
8. Kim N, Kim JJ, Choe YH, Kim HS, Kim JI, Chung IS. Korean College of Helicobacter and Upper Gastrointestinal Research; Korean Association of Gastroenterology. Diagnosis and treatment guidelines for Helicobacter pylori infection in Korea. Korean J Gastroenterol. 2009; 54:269–278.
9. Asaka M, Kato M, Takahashi S, et al. Japanese Society for Helicobacter Research. Guidelines for the management of Helicobacter pylori infection in Japan:2009 revised edition. Helicobacter. 2010; 15:1–20.
10. Kato M, Asaka M. Recent development of gastric cancer prevention. Jpn J Clin Oncol. 2012; 42:987–994.

11. Kim SG, Song HJ, Choi IJ, et al. Korean College of Helicobacter, Upper Gastrointestinal Research. Helicobacter pylori eradication on iatrogenic ulcer by endoscopic resection of gastric tumour: a prospective, randomized, placebo-controlled multicentre trial. Dig Liver Dis. 2013; 45:385–389.
12. Fukase K, Kato M, Kikuchi S, et al. Japan Gast Study Group. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008; 372:392–397.
13. Take S, Mizuno M, Ishiki K, et al. The long-term risk of gastric cancer after the successful eradication of Helicobacter pylori. J Gastroenterol. 2011; 46:318–324.
14. Yanaoka K, Oka M, Ohata H, et al. Eradication of Helicobacter pylori prevents cancer development in subjects with mild gastric atrophy identified by serum pepsinogen levels. Int J Cancer. 2009; 125:2697–2703.
15. Ogura K, Hirata Y, Yanai A, et al. The effect of Helicobacter pylori eradication on reducing the incidence of gastric cancer. J Clin Gastroenterol. 2008; 42:279–283.
16. Takenaka R, Okada H, Kato J, et al. Helicobacter pylori eradication reduced the incidence of gastric cancer, especially of the intestinal type. Aliment Pharmacol Ther. 2007; 25:805–812.
17. Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001; 345:784–789.
18. Kang JM, Kim N, Shin CM, et al. Predictive factors for improvement of atrophic gastritis and intestinal metaplasia after Helicobacter pylori eradication: a three-year follow-up study in Korea. Helicobacter. 2012; 17:86–95.
19. Shin CM, Kim N, Yang HJ, et al. Stomach cancer risk in gastric cancer relatives: interaction between Helicobacter pylori infection and family history of gastric cancer for the risk of stomach cancer. J Clin Gastroenterol. 2010; 44:e34–e39.
20. Nam JH, Choi IJ, Cho SJ, et al. Helicobacter pylori infection and histological changes in siblings of young gastric cancer patients. J Gastroenterol Hepatol. 2011; 26:1157–1163.
21. Chang YW, Han YS, Lee DK, et al. Role of Helicobacter pylori infection among offspring or siblings of gastric cancer patients. Int J Cancer. 2002; 101:469–474.
22. Jung JH, Choi KD, Han S, et al. Seroconversion rates of Helicobacter pylori infection in Korean adults. Helicobacter. 2013; 18:299–308.
23. Suzuki H, Moayyedi P. Helicobacter pylori infection in functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013; 10:168–174.
24. Kim SE, Park YS, Kim N, et al. Effect of Helicobacter pylori eradication on functional dyspepsia. J Neurogastroenterol Motil. 2013; 19:233–243.
25. Miyamoto M, Haruma K. Gastric ulcer and duodenal ulcer. Nihon Rinsho. 2013; 71:1418–1423.
26. Song HJ, Kwon JW, Kim N, Park YS. Cost effectiveness associated with Helicobacter pylori screening and eradication in patients taking nonsteroidal anti-inflammatory drugs and/or aspirin. Gut Liver. 2013; 7:182–189.
27. Asaka M, Kato M, Sakamoto N. Roadmap to eliminate gastric cancer with Helicobacter pylori eradication and consecutive surveillance in Japan. J Gastroenterol. 2014; 49:1–8.
28. Tanaka A, Tokunaga K, Takahashi S. The practical assessment of H. pylori eradication. Nihon Rinsho. 2013; 71:1388–1393.
29. Furuta T, Sugimoto M, Uotani T, Sahara S, Ichikawa H. Present status and future prospects of third line rescue regimens for H. pylori infection. Nihon Rinsho. 2013; 71:1404–1412.
30. Lee BH, Kim N, Hwang TJ, et al. Bismuth-containing quadruple therapy as second-line treatment for Helicobacter pylori infection: effect of treatment duration and antibiotic resistance on the eradication rate in Korea. Helicobacter. 2010; 15:38–45.
31. Horiki N, Omata F, Uemura M, et al. Annual change of primary resistance to clarithromycin among Helicobacter pylori isolates from 1996 through 2008 in Japan. Helicobacter. 2009; 14:86–90.
32. Murakami K, Furuta T, Ando T, et al. Japan GAST Study Group. Multicenter randomized controlled study to establish the standard third-line regimen for Helicobacter pylori eradication in Japan. J Gastroenterol. 2013; 48:1128–1135.
33. Chung JW, Ha M, Yun SC, et al. Meta-analysis: sequential therapy is superior to conventional therapy for Helicobacter pylori infection in Korea. Korean J Gastroenterol. 2013; 62:267–271.
34. Lee JW, Kim N, Kim JM, et al. Prevalence of primary and secondary antimicrobial resistance of Helicobacter pylori in Korea from 2003 through 2012. Helicobacter. 2013; 18:206–214.
35. Lee JH, Kim N, Chung JI, et al. Longterm follow up of Helicobacter pylori IgG serology after eradication and reinfection rate of H. pylori in South Korea. Helicobacter. 2008; 13:288–294.
36. Take S, Mizuno M, Ishiki K, et al. Reinfection rate of Helicobacter pylori after eradication treatment: a long-term prospective study in Japan. J Gastroenterol. 2012; 47:641–646.
37. Kim MS, Kim N, Kim SE, et al. Longterm follow-up Helicobacter pylori reinfection rate and its associated factors in Korea. Helicobacter. 2013; 18:135–142.
38. Okimoto T, Murakami K, Sato R, et al. Is the recurrence of Helicobacter pylori infection after eradication therapy resultant from recrudescence or reinfection, in Japan. Helicobacter. 2003; 8:186–191.
39. Choi J, Kim SG, Yoon H, et al. Eradication of Helicobacter pylori after endoscopic resection of gastric tumors does not reduce incidence of metachronous gastric carcinoma. Clin Gastroenterol Hepatol. 2013. DOI: doi:10.1016/j.cgh.2013.09.057. [Epub ahead of print].
40. Bae SE, Jung HY, Kang J, et al. Effect of Helicobacter pylori eradication on metachronous recurrence after endoscopic resection of gastric neoplasm. Am J Gastroenterol. 2014; 109:60–67.
41. Chon I, Choi C, Shin CM, Park YS, Kim N, Lee DH. Effect of Helicobacter pylori eradication on subsequent dysplasia development after endoscopic resection of gastric dysplasia. Korean J Gastroenterol. 2013; 61:307–312.
42. Cho SJ, Choi IJ, Kook MC, et al. Randomised clinical trial: the effects of Helicobacter pylori eradication on glandular atrophy and intestinal metaplasia after subtotal gastrectomy for gastric cancer. Aliment Pharmacol Ther. 2013; 38:477–489.
43. Nam SY, Choi IJ, Ryu KH, Kim BC, Kim CG, Nam BH. Effect of Helicobacter pylori infection and its eradication on reflux esophagitis and reflux symptoms. Am J Gastroenterol. 2010; 105:2153–2162.
44. Kim N, Lee SW, Kim JI, et al. H pylori and GERD Study Group of Korean College of Helicobacter and Upper Gastrointestinal Research. Effect of Helicobacter pylori eradication on the development of reflux esophagitis and gastroesophageal reflux symptoms: a nationwide multicenter prospective study. Gut Liver. 2011; 5:437–446.
45. Take S, Mizuno M, Ishiki K, et al. Helicobacter pylori eradication may induce de novo, but transient and mild, reflux esophagitis: Prospective endoscopic evaluation. J Gastroenterol Hepatol. 2009; 24:107–113.
46. Hirata K, Suzuki H, Matsuzaki J, et al. Improvement of reflux symptom related quality of life after Helicobacter pylori eradication therapy. J Clin Biochem Nutr. 2013; 52:172–178.
47. Minatsuki C, Yamamichi N, Shimamoto T, et al. Background factors of reflux esophagitis and non-erosive reflux disease: a cross-sectional study of 10,837 subjects in Japan. PLoS One. 2013; 8:e69891.

48. Lee ES, Yoon YS, Park CY, et al. Eradication of Helicobacter pylori increases ghrelin mRNA expression in the gastric mucosa. J Korean Med Sci. 2010; 25:265–271.
49. Jang EJ, Park SW, Park JS, et al. The influence of the eradication of Helicobacter pylori on gastric ghrelin, appetite, and body mass index in patients with peptic ulcer disease. J Gastroenterol Hepatol. 2008; 23(Suppl 2):S278–S285.
50. Ando T, Ishikawa T, Takagi T, et al. Impact of Helicobacter pylori eradication on circulating adiponectin in humans. Helicobacter. 2013; 18:158–164.
Go to : 
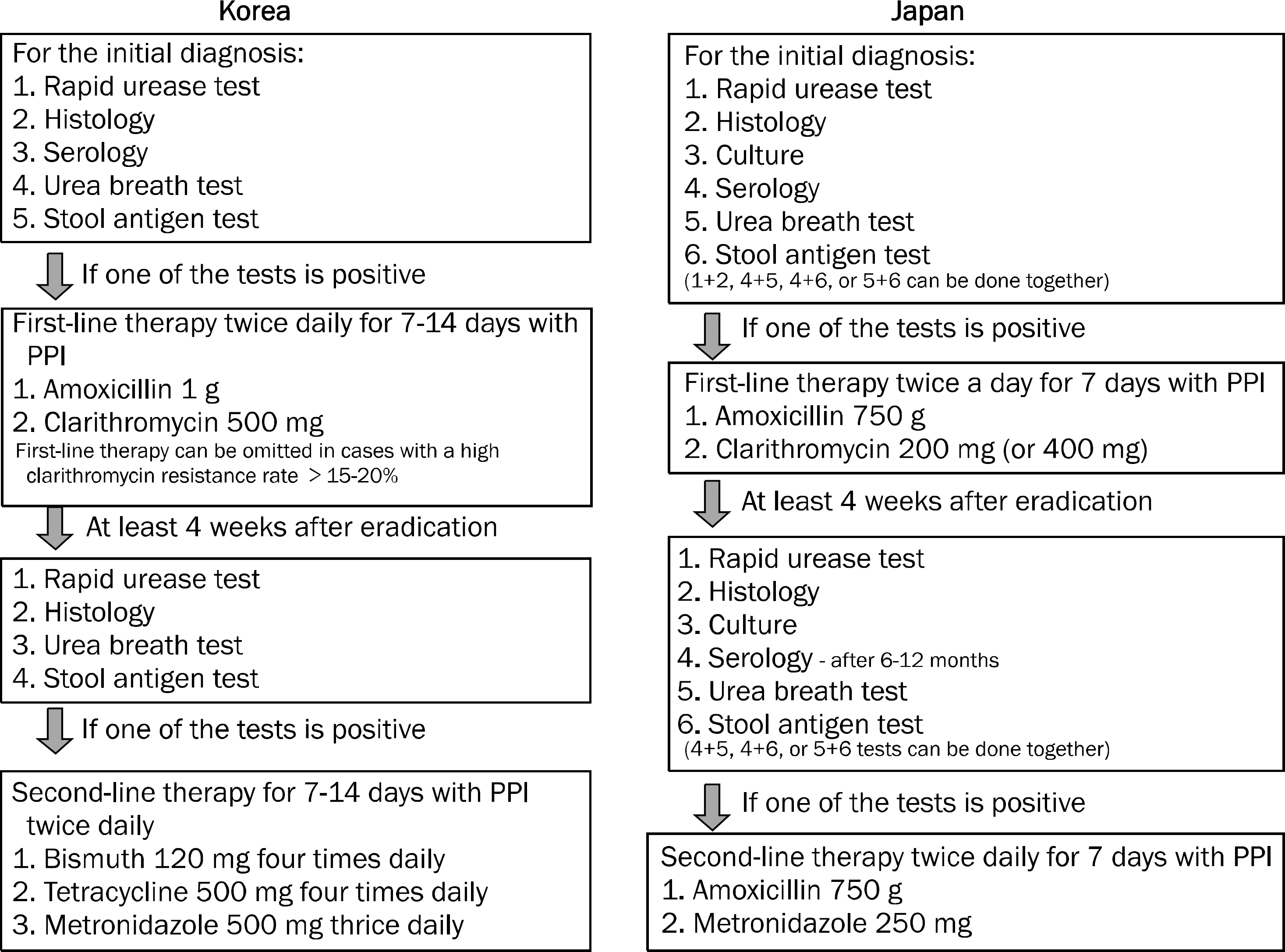 | Fig. 1.Diagnostic and therapeutic methods for Helicobacter pylori infection in 2013 guidelines. Bacterial culture is not recommended as a diagnostic method in Korea, while either two noninvasive tests or one invasive test is recommended in Japan. Notably, a decrease relative to the initial serum antibody level of more than 50% after 6–12 months is considered the most reliable method in Japan for determining successful eradication. With regard to the treatment, first-line treatment can be skip-ped and move directly to second-line treatment using bismuth-based quadruple therapy in cases with a high clarithromycin resistance rate in Korea, whereas neither 14 days nor bismuth-based regimen is recommended in Japan. The Japanese guideline recommends lower dose of antibiotics for shorter duration (7 days) for eradication. PPI, proton pump inhibitor. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download