Abstract
Background/Aims
Biliary drainage is performed in many patients with cholangiocarcinoma (CCA) to relieve obstructive jaundice. For those who have undergone biliary drainage, bile cytology can be easily performed since the access is already achieved. This study aims to determine the clinical usefulness of bile cytology for the diagnosis of CCA and to evaluate factors affecting its diagnostic yield.
Methods
A total of 766 consecutive patients with CCA underwent bile cytology via endoscopic nasobiliary drainage or percutaneous transhepatic biliary drainage from January 2000 to June 2012. Data were collected by retrospectively reviewing the medical records. We evaluated the diagnostic yield of bile cytology with/without other sampling methods including brush cytology and endobiliary forcep biopsy, and the optimal number of repeated bile sampling. Several factors affecting diagnostic yield were then analyzed.
Results
The sensitivity of bile cytology, endobiliary forceps biopsy, and a combination of both sampling methods were 24.7% (189/766), 74.4% (259/348), and 77.9% (271/348), respectively. The cumulative positive rate of bile sampling increased from 40.7% (77/189) at first sampling to 93.1% (176/189) at third sampling. On multivariate analysis, factors associated with positive bile cytology were perihilar tumor location, intraductal growing tumor type, tumor extent ≥20 mm, poorly differentiated grade tumor, and three or more samplings.
Go to : 
References
1. Khan SA, Davidson BR, Goldin RD, et al. British Society of Gastroenterology. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012; 61:1657–1669.

2. Coelho-Prabhu N, Baron TH. Endoscopic retrograde cholangiopancreatography in the diagnosis and management of cholangiocarcinoma. Clin Liver Dis. 2010; 14:333–348.

3. Glasbrenner B, Ardan M, Boeck W, Preclik G, Möller P, Adler G. Prospective evaluation of brush cytology of biliary strictures during endoscopic retrograde cholangiopancreatography. Endoscopy. 1999; 31:712–717.

4. Ponchon T, Gagnon P, Berger F, et al. Value of endobiliary brush cytology and biopsies for the diagnosis of malignant bile duct stenosis: results of a prospective study. Gastrointest Endosc. 1995; 42:565–572.

5. Pugliese V, Conio M, Nicolò G, Saccomanno S, Gatteschi B. Endoscopic retrograde forceps biopsy and brush cytology of biliary strictures: a prospective study. Gastrointest Endosc. 1995; 42:520–526.

6. Ohshima Y, Yasuda I, Kawakami H, et al. EUS-FNA for suspected malignant biliary strictures after negative endoscopic transpapillary brush cytology and forceps biopsy. J Gastroenterol. 2011; 46:921–928.

7. Tummala P, Munigala S, Eloubeidi MA, Agarwal B. Patients with obstructive jaundice and biliary stricture ± mass lesion on imaging: prevalence of malignancy and potential role of EUS-FNA. J Clin Gastroenterol. 2013; 47:532–537.
8. Barr Fritcher EG, Kipp BR, Slezak JM, et al. Correlating routine cytology, quantitative nuclear morphometry by digital image analysis, and genetic alterations by fluorescence in situ hybridization to assess the sensitivity of cytology for detecting pan-creatobiliary tract malignancy. Am J Clin Pathol. 2007; 128:272–279.

9. Harewood GC, Baron TH, Stadheim LM, Kipp BR, Sebo TJ, Salomao DR. Prospective, blinded assessment of factors influencing the accuracy of biliary cytology interpretation. Am J Gastroenterol. 2004; 99:1464–1469.

10. Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma. Clin Gastroenterol Hepatol. 2013; 11:13–21.e1.

11. Liver Cancer Study Group of Japan. The general rules for the clinical and pathological study of primary liver cancer. Jpn J Surg. 1989; 19:98–129.
12. Lim JH. Cholangiocarcinoma: morphologic classification according to growth pattern and imaging findings. AJR Am J Roentgenol. 2003; 181:819–827.

13. Sasaki A, Aramaki M, Kawano K, et al. Intrahepatic peripheral cholangiocarcinoma: mode of spread and choice of surgical treatment. Br J Surg. 1998; 85:1206–1209.

14. Mansfield JC, Griffin SM, Wadehra V, Matthewson K. A prospective evaluation of cytology from biliary strictures. Gut. 1997; 40:671–677.

15. Foutch PG, Kerr DM, Harlan JR, Kummet TD. A prospective, controlled analysis of endoscopic cytotechniques for diagnosis of malignant biliary strictures. Am J Gastroenterol. 1991; 86:577–580.
16. Davidson B, Varsamidakis N, Dooley J, et al. Value of exfoliative cytology for investigating bile duct strictures. Gut. 1992; 33:1408–1411.

17. Sugiyama M, Atomi Y, Wada N, Kuroda A, Muto T. Endoscopic transpapillary bile duct biopsy without sphincterotomy for diagnosing biliary strictures: a prospective comparative study with bile and brush cytology. Am J Gastroenterol. 1996; 91:465–467.
18. Mohandas KM, Swaroop VS, Gullar SU, Dave UR, Jagannath P, DeSouza LJ. Diagnosis of malignant obstructive jaundice by bile cytology: results improved by dilating the bile duct strictures. Gastrointest Endosc. 1994; 40:150–154.

19. Kurzawinski TR, Deery A, Dooley JS, Dick R, Hobbs KE, Davidson BR. A prospective study of biliary cytology in 100 patients with bile duct strictures. Hepatology. 1993; 18:1399–1403.

20. Abdelghani YA, Arisaka Y, Masuda D, et al. Bile aspiration cytology in diagnosis of bile duct carcinoma: factors associated with positive yields. J Hepatobiliary Pancreat Sci. 2012; 19:370–378.

21. Yagioka H, Hirano K, Isayama H, et al. Clinical significance of bile cytology via an endoscopic nasobiliary drainage tube for pathological diagnosis of malignant biliary strictures. J Hepatobiliary Pancreat Sci. 2011; 18:211–215.

22. Hattori M, Nagino M, Ebata T, Kato K, Okada K, Shimoyama Y. Prospective study of biliary cytology in suspected perihilar cholangiocarcinoma. Br J Surg. 2011; 98:704–709.

23. Uchida N, Kamada H, Ono M, et al. How many cytological exami-nations should be performed for the diagnosis of malignant biliary stricture via an endoscopic nasobiliary drainage tube? J Gastroenterol Hepatol. 2008; 23:1501–1504.

24. Igami T, Nagino M, Oda K, et al. Clinicopathologic study of cholangiocarcinoma with superficial spread. Ann Surg. 2009; 249:296–302.

26. Polkowski M, Larghi A, Weynand B, et al. European Society of Gastrointestinal Endoscopy (ESGE). Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy. 2012; 44:190–206.

27. Athanassiadou P, Grapsa D. Value of endoscopic retrograde cholangiopancreatography-guided brushings in preoperative assessment of pancreaticobiliary strictures: what's new? Acta Cytol. 2008; 52:24–34.
28. Yamaguchi K, Nakano K, Nagai E, et al. Ki-ras mutations in codon 12 and p53 mutations (biomarkers) and cytology in bile in patients with hepatobiliary-pancreatic carcinoma. Hepatogastroenterology. 2005; 52:713–718.
Go to : 
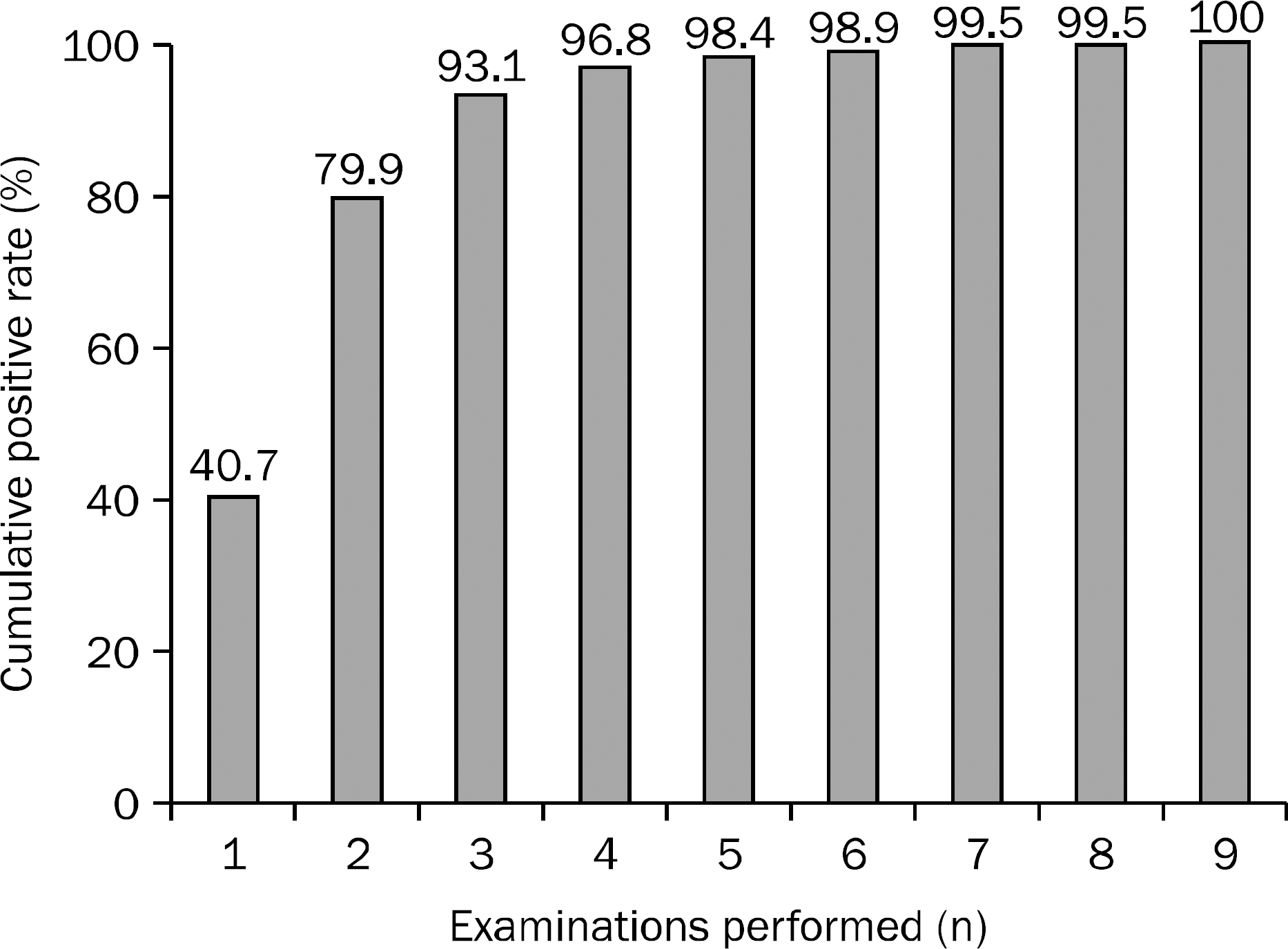 | Fig. 1.The cumulative positive rates of bile cytology examination. In 189 patients with positive cytology results, the cumulative positive rate rose to 93.1% (176/189) at third examination. |
Table 1.
Patients Characteristics
Table 2.
Sensitivities of Each Diagnostic Methods and Combined Approaches
Table 3.
All Variables Studied for Positive Bile Cytology
Table 4.
Multivariate Analysis on Factors Affecting Diagnostic Yield of Bile Cytology




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download