Abstract
Background/Aims
Serrated adenomas of the colon show mixed characteristics of both hyperplastic and adenomatous polyps. Serrated adenomas are known to progress via the serrated pathway than the adenoma-carcinoma pathway. The aim of this study was to evaluate the characteristics of traditional serrated adenomas compared to hyperplastic polyps and tubular adenomas by using immunohistochemical staining for p53, Bcl-2, and Ki-67.
Methods
Age, sex, location, size and the immunoexpression of p53, Bcl-2, and Ki-67 were retrospectively analyzed in 20 traditional serrated adenomas, 20 hyperplastic polyps, and 20 tubular adenomas from January 2007 to December 2012 at The Catholic University of Korea, Yeouido St. Mary's Hospital.
Results
There was no difference in Bcl-2 and p53 expression between traditional serrated adenomas and hyperplastic polyps. Ki-67 Expression of traditional serrated adenomas was higher than that of hyperplastic polyps (p=0.001). Ki-67 and p53 expression was similar between traditional serrated and tubular adenomas. Bcl-2 expression of traditional serrated adenomas was lower than that of tubular adenomas (p=0.001). Regarding the expression of p53, Bcl-2, and Ki-67 in traditional serrated adenomas, there were no statistical differences among age, sex, location, and size.
Conclusions
Our study suggested that Ki-67 may be helpful in distinguishing traditional serrated adenomas from hyperplastic polyps, and p53 expression may be ineffective in distinguishing between traditional serrated and tubular adenomas. From Bcl-2 expression, it is suggested that the tumorigenesis of traditional serrated adenomas is lower than that of tubular adenomas.
Go to : 
References
1. Mäkinen MJ, George SM, Jernvall P, Mäkelä J, Vihko P, Karttunen TJ. Colorectal carcinoma associated with serrated adenoma–prevalence, histological features, and prognosis. J Pathol. 2001; 193:286–294.
2. Longacre TA, Fenoglio-Preiser CM. Mixed hyperplastic adenomatous polyps/serrated adenomas. A distinct form of colorectal neoplasia. Am J Surg Pathol. 1990; 14:524–537.
3. Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010; 138:2088–2100.

4. Hong SN, Yang DH, Kim YH, et al. MultiSociety Task Force for Development of Guidelines for Colorectal Polyp Screening, Surveillance and Management. Korean guidelines for post-polypectomy colonoscopic surveillance. Korean J Gastroenterol. 2012; 59:99–117.

5. Terdiman JP, McQuaid KR. Surveillance guidelines should be updated to recognize the importance of serrated polyps. Gastroenterology. 2010; 139:1444–1447.

6. Jass JR. Serrated adenoma of the colorectum: a lesion with teeth. Am J Pathol. 2003; 162:705–708.
7. Pap Z, Pávai Z, Dénes L, Brînzaniuc K, Jung I. Hyperplastic polyps and serrated adenomas: precancerous lesions with mixed immunophenotype. Rom J Morphol Embryol. 2011; 52:797–802.
8. Jass JR, Baker K, Zlobec I, et al. Advanced colorectal polyps with the molecular and morphological features of serrated polyps and adenomas: concept of a ‘fusion' pathway to colorectal cancer. Histopathology. 2006; 49:121–131.

9. Snover DC, Jass JR, Fenoglio-Preiser C, Batts KP. Serrated polyps of the large intestine: a morphologic and molecular review of an evolving concept. Am J Clin Pathol. 2005; 124:380–391.
10. Takayama T, Miyanishi K, Hayashi T, Sato Y, Niitsu Y. Colorectal cancer: genetics of development and metastasis. J Gastroenterol. 2006; 41:185–192.

11. Ozawa A, Konishi F, Fukayama M, Kanazawa K. Apoptosis and its regulation in flat-type early colorectal carcinoma: comparison with that in polypoid-type early colorectal carcinoma. Dis Colon Rectum. 2000; 43(10 Suppl):S23–S28.
12. Purdie CA, O'Grady J, Piris J, Wyllie AH, Bird CC. p53 expression in colorectal tumors. Am J Pathol. 1991; 138:807–813.
13. Kaklamanis L, Gatter KC, Mortensen N, et al. p53 expression in colorectal adenomas. Am J Pathol. 1993; 142:87–93.
14. Ladas SD, Kitsanta P, Triantafyllou K, Tzathas C, Spiliadi C, Raptis SA. Cell turnover of serrated adenomas. J Pathol. 2005; 206:62–67.

15. Sinicrope FA, Ruan SB, Cleary KR, Stephens LC, Lee JJ, Levin B. bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995; 55:237–241.
16. Popescu RA, Lohri A, de Kant E, et al. bcl-2 expression is reciprocal to p53 and c-myc expression in metastatic human colorectal cancer. Eur J Cancer. 1998; 34:1268–1273.

17. Sawyer EJ, Cerar A, Hanby AM, et al. Molecular characteristics of serrated adenomas of the colorectum. Gut. 2002; 51:200–206.

18. Hiyama T, Yokozaki H, Shimamoto F, et al. Frequent p53 gene mutations in serrated adenomas of the colorectum. J Pathol. 1998; 186:131–139.
19. Daniel P, Wagrowska-Danilewicz M, Danilewicz M, Stasikowska O, Malecka-Panas E. Transforming growth factor beta 1 and metalloproteinase-9 overexpression in colorectal cancer (CC) and adenoma. Int J Colorectal Dis. 2007; 22:1165–1172.

20. Fujimori Y, Fujimori T, Imura J, et al. An assessment of the diagnostic criteria for sessile serrated adenoma/polyps: SSA/Ps using image processing software analysis for Ki67 immunohistochemistry. Diagn Pathol. 2012; 7:59.

21. Choi KW, Jang BI, Eun JR, Lee JH, Bae YK, Kim TN. Expression of β-catenin, hMLH1, p53, Bcl-2, Bax and COX-2 in serrated adenomas of colon. Korean J Gastrointest Endosc. 2007; 34:19–27.
22. Bellizzi AM, Rock J, Marsh WL, Frankel WL. Serrated lesions of the appendix: a morphologic and immunohistochemical appraisal. Am J Clin Pathol. 2010; 133:623–632.
23. Fu B, Yachida S, Morgan R, Zhong Y, Montgomery EA, IacobuzioDonahue CA. Clinicopathologic and genetic characterization of traditional serrated adenomas of the colon. Am J Clin Pathol. 2012; 138:356–366.

24. Harvey NT, Ruszkiewicz A. Serrated neoplasia of the colorectum. World J Gastroenterol. 2007; 13:3792–3798.

25. Cunningham KS, Riddell RH. Serrated mucosal lesions of the colorectum. Curr Opin Gastroenterol. 2006; 22:48–53.

26. Krajewska M, Fenoglio-Preiser CM, Krajewski S, et al. Immunohistochemical analysis of Bcl-2 family proteins in adenocarcinomas of the stomach. Am J Pathol. 1996; 149:1449–1457.
27. Yao T, Kouzuki T, Kajiwara M, Matsui N, Oya M, Tsuneyoshi M. ‘Serrated' adenoma of the colorectum, with reference to its gastric differentiation and its malignant potential. J Pathol. 1999; 187:511–517.

28. Kim HJ, Kim TH, Lim BL, et al. Clinical Features of Colorectal Serrated Adenomas. J Korean Soc Coloproctol. 2006; 22:91–96.
29. Hawkins NJ, Bariol C, Ward RL. The serrated neoplasia pathway. Pathology. 2002; 34:548–555.
Go to : 
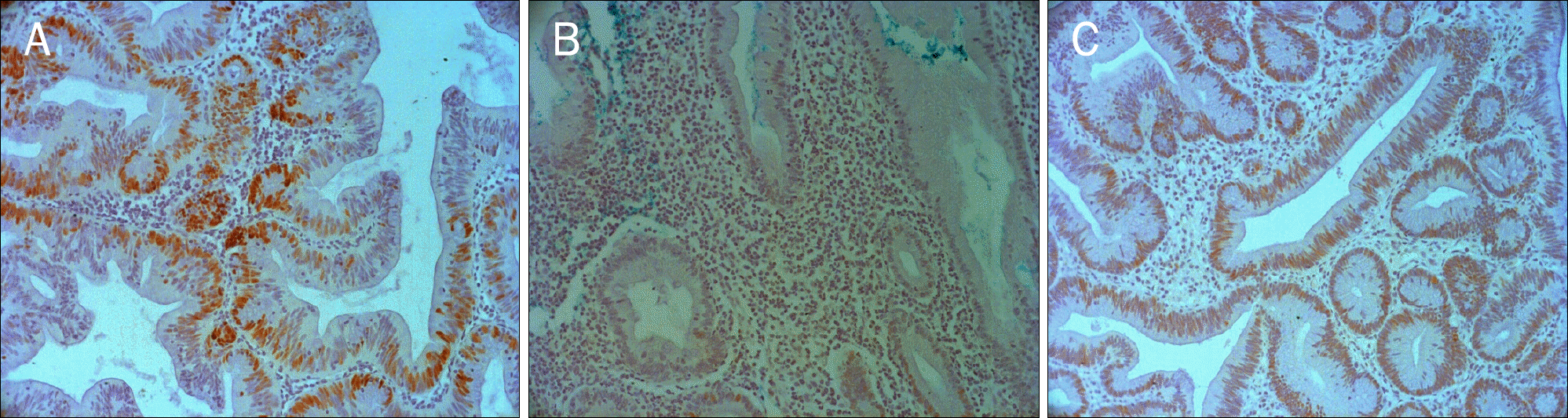 | Fig. 1.Immunohistochemical staining of p53 in traditional serrated adenoma, hyperplastic polyp, and tubular adenoma. Intense nuclear staining of p53 is seen in traditional serrated adenoma (A, ×200) and tubular adenoma (C, ×200). However, p53 protein overexpression is not observed in hyperplastic polyp (B, ×200). |
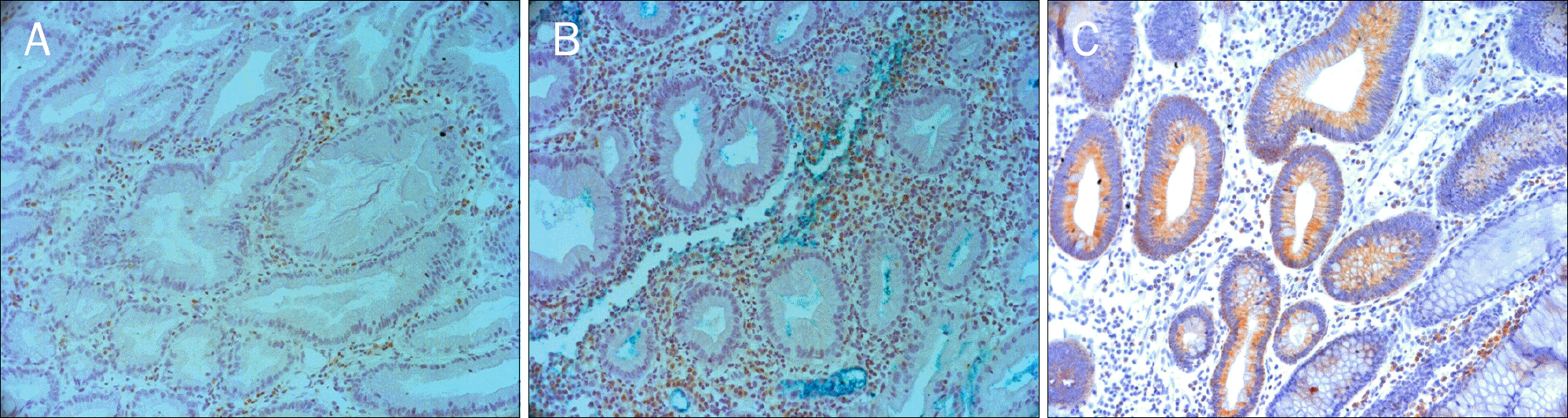 | Fig. 2.Immunohistochemical staining of Bcl-2 in traditional serrated adenoma, hyperplastic polyp, and tubular adenoma. Traditional serrated adenoma (A, ×200) and hyperplastic polyp (B, ×200) show loss of Bcl-2 expression. Tubular adenoma (C, ×200) shows cytoplasmic immunoreactivity for Bcl-2. Small lymphocytes in the lamina propria are internal control of Bcl-2 immunostaining. |
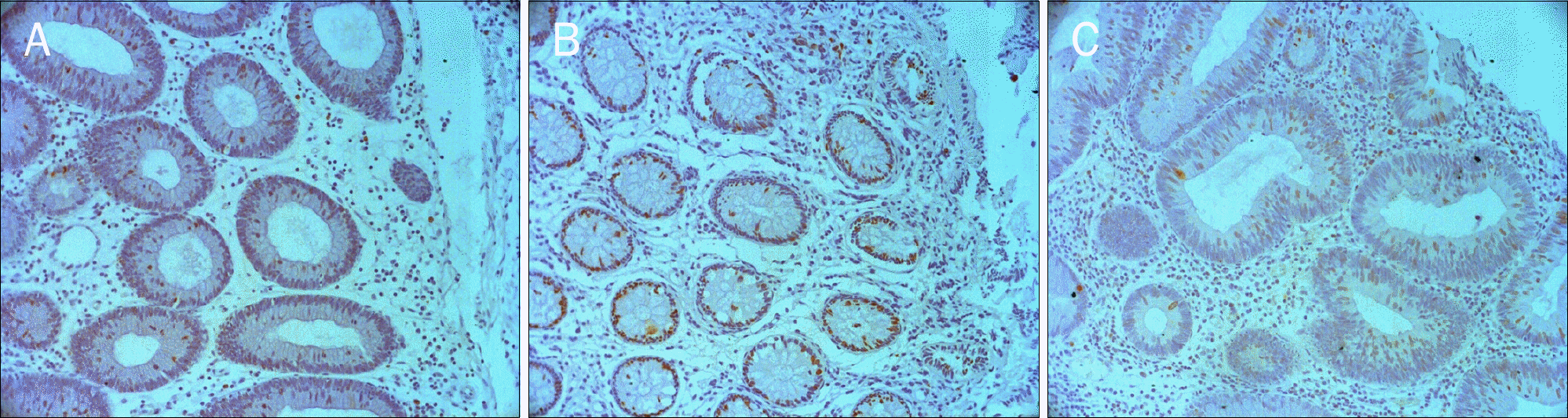 | Fig. 3.Immunohistochemical staining of Ki-67 in traditional serrated adenoma, hyperplastic polyp, and tubular adenoma. Ki-67 positive cells (brown color) are seen at the bottom and on the surface of individual crypts of traditional serrated adenoma (A, ×200), hyperplastic polyp (B, ×200), and tubular adenoma (C, ×200). |
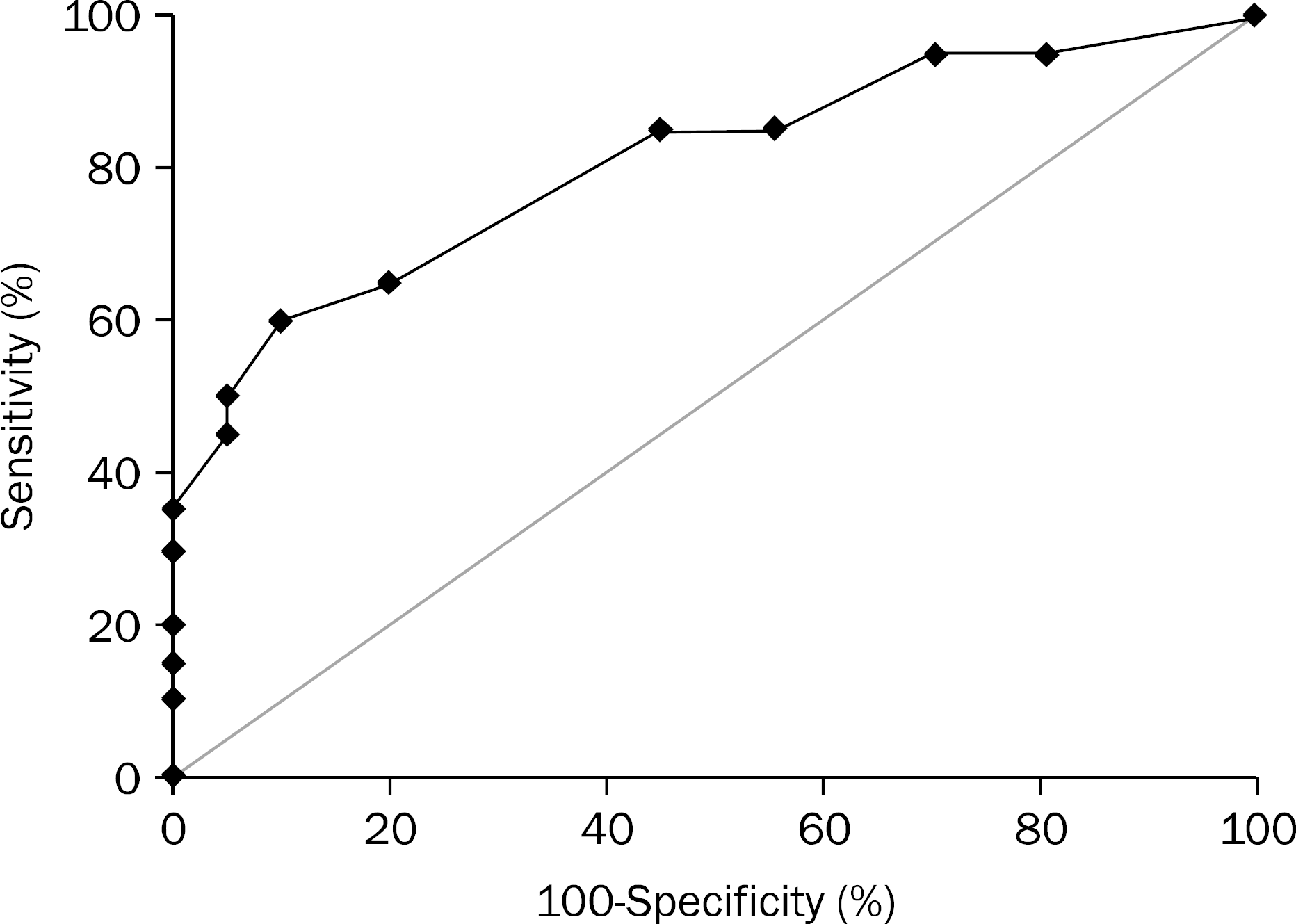 | Fig. 4.Receiver operating characteristic analysis of Ki-67 level to identify traditional serrated adenomas. Area under the curve (AUC) was 0.808 (95% CI, 0.672–0.943) with cutoff point of 6%. Sensitivity=65%, specificity=80%. |
Table 1.
Clinicopathologic Features of Patients with Traditional Serrated Adenomas, Tubular Adenomas, and Hyperplastic Polyps
Table 2.
Expressions of p53, Bcl-2, Ki-67 in Patients with Traditional Serrated Adenomas, Tubular Adenomas, and Hyperplastic Polyps
Table 3.
Relationship between Clinicopathological Features and Expressions of p53, Bcl-2, Ki-67 in Traditional Serrated Adenomas
Table 4.
Expressions of p53, Bcl-2, Ki-67 in Traditional Serrated Adenomas with Low and High Grade Dysplasia
Table 5.
Expressions of p53, Bcl-2, Ki-67 in Tubular Adenomas with Low and High Grade Dysplasia




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download