Abstract
Background/Aims
Gastric lymphoepithelioma-like carcinoma (LLC) is a rare cancer that presents with a unique histologic pattern that is characterized by poorly differentiated malignant cells infiltrating the background stroma along with massive lymphocyte infiltration. Many studies have shown that gastric LLC is associated with better prognosis than other gastric malignancies. However, the reason for better prognosis has not been clarified and the underlying mechanism remains to be elucidated. Therefore, we attempted to determine the clinical characteristics of gastric LLC and identify its prognostic factors related to improved survival.
Methods
A total of 18 patients were diagnosed with gastric LLC after resection from 2005 to 2012 at Department of Gastroenterology in Chungnam National University Hospital. The data of these patients were compared with 36 age- and sex-match-ed patients with poorly differentiated gastric adenocarcinoma who also underwent resection during the same study period.
Results
Postoperative recurrence or metastasis tended to occur less frequent in gastric LLC than in poorly-differentiated gastric adenocarcinoma. Among prognostic factors, only the number of lymph node metastases showed significant difference, with gastric LLC being associated with a smaller number of lymph node metastases. Regarding the disease free and overall survival rate, both were higher for gastric LLC than for poorly-differentiated gastric adenocarcinoma, albeit not statistically significant (p=0.089 and p=0.159, respectively).
Go to : 
References
1. Wang HH, Wu MS, Shun CT, Wang HP, Lin CC, Lin JT. Lymphoepithelioma-like carcinoma of the stomach: a subset of gastric carcinoma with distinct clinicopathological features and high prevalence of Epstein-Barr virus infection. Hepatogastroenterology. 1999; 46:1214–1219.
2. MacCarty WC, Mahle AE. Relation of differentiation and lymphocytic infiltration to postoperative longevity in gastric carcinoma. J Lab Clin Med. 1921; 6:473–480.
3. Steiner PE, Maimon SN, Palmer WL, Kirsner JB. Gastric cancer; morphologic factors in 5-year survival after gastrectomy. Am J Pathol. 1948; 24:947–969.
4. Hamazaki M, Sawayama K, Kuriya T. Stomach cancer with lymphoid stroma. J Karyopathol. 1968; 12:115–120.
5. Watanabe H, Enjoji M, Imai T. Gastric carcinoma with lymphoid stroma. Its morphologic characteristics and prognostic correlations. Cancer. 1976; 38:232–243.
6. Minamoto T, Mai M, Watanabe K, et al. Medullary carcinoma with lymphocytic infiltration of the stomach. Clinicopathologic study of 27 cases and immunohistochemical analysis of the subpopulations of infiltrating lymphocytes in the tumor. Cancer. 1990; 66:945–952.

7. Lertprasertsuke N, Tsutsumi Y. Gastric carcinoma with lymphoid stroma. Analysis using mucin histochemistry and immunohistochemistry. Virchows Arch A Pathol Anat Histopathol. 1989; 414:231–241.
8. Cho JH, Lee WS, Lee KR, et al. Gastric lymphoepithelioma-like carcinoma diagnosed and treated by endoscopic submucosal dissection: review of the literature. Korean J Gastrointest Endosc. 2010; 40:256–260.
9. Woo H, Shin SJ, Kim YB, et al. A case of endoscopic enucleation for gastric lymphoepithelioma-like carcinoma. Korean J Gastrointest Endosc. 2010; 41:163–167.
10. Park WI, Kim HW, Park JH, et al. A case of gastric lymphoepithelio-ma-like carcinoma presenting as a submucosal tumor. Korean J Gastrointest Endosc. 2004; 28:123–126.
11. Oda K, Tamaru J, Takenouchi T, et al. Association of Epstein-Barr virus with gastric carcinoma with lymphoid stroma. Am J Pathol. 1993; 143:1063–1071.
Go to : 
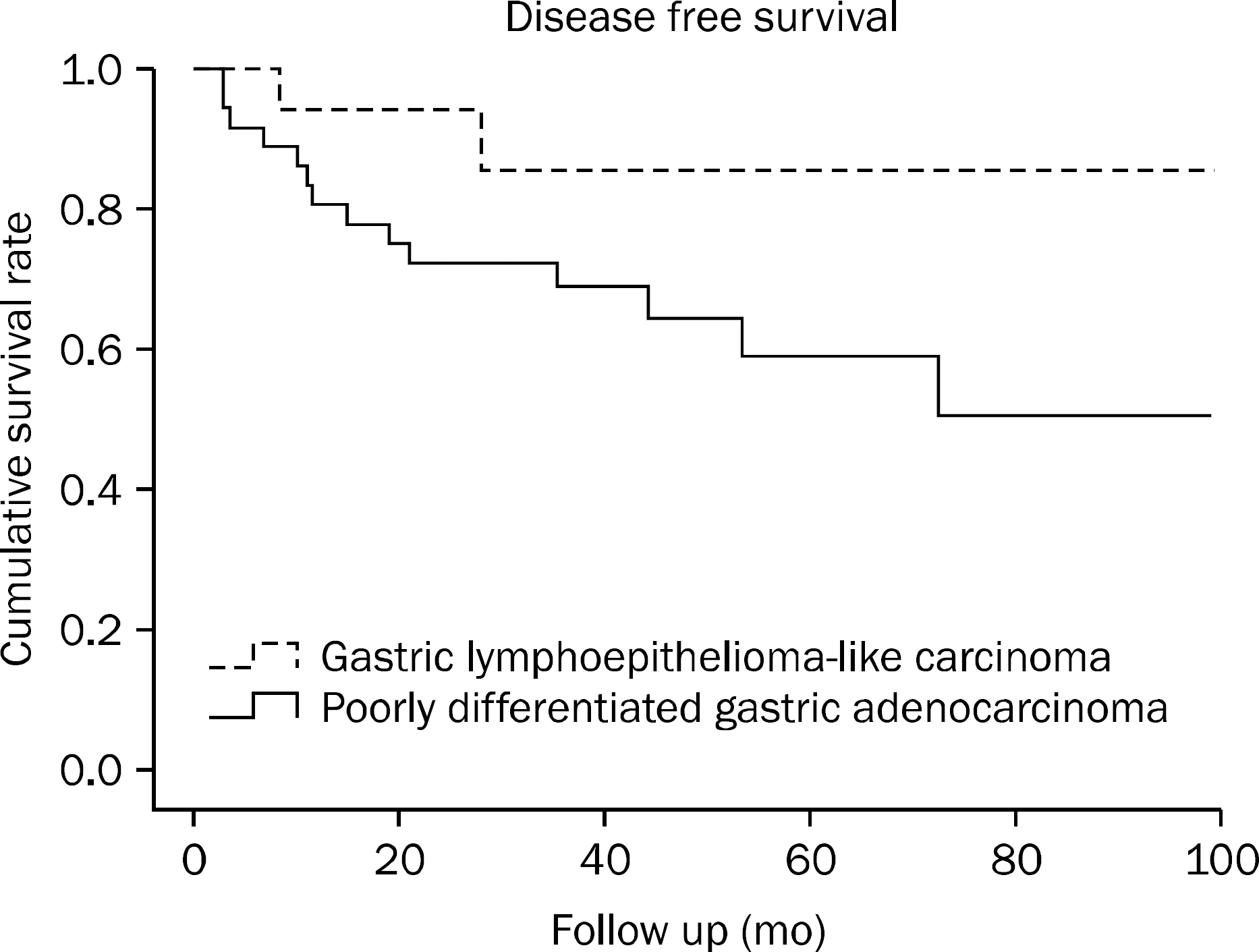 | Fig. 1.Disease free survival rate of patients with gastric lympho-epithelioma-like carcinoma and poorly differentiated gastric adenocarcinoma. The disease free survival rate was higher among patients with gastric lymphoepithelioma-like carcinoma than among those with poorly differentiated gastric adenocarcinoma, although this difference was not statistically significant (p=0.089). |
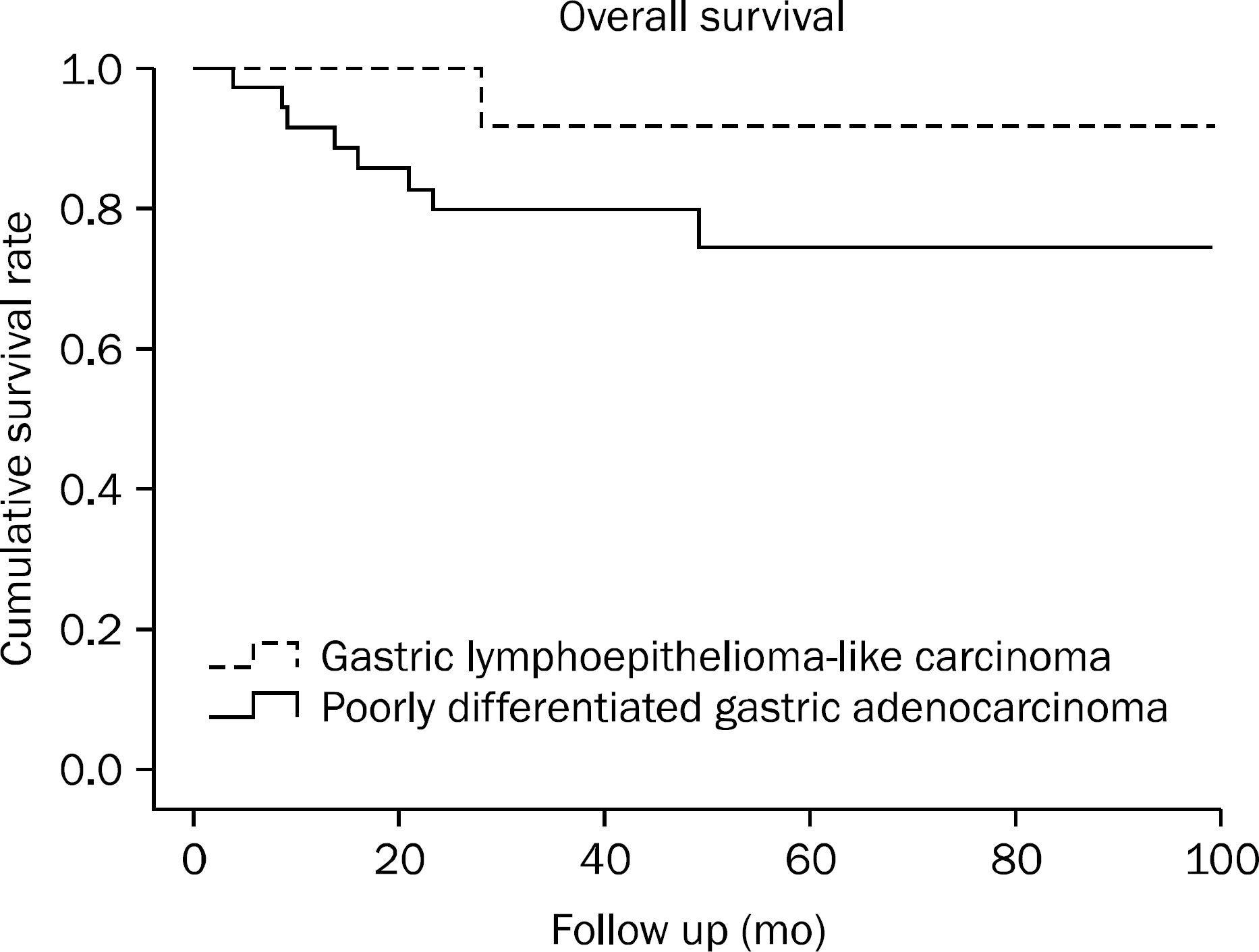 | Fig. 2.Overall survival rate of patients with gastric lympho-epithelioma-like carcinoma and poorly differentiated gastric adeno-carcinoma. The overall survival rate was higher among patients with gastric lymphoepithelioma-like carcinoma than among those with poorly differentiated gastric adenocarcinoma, although this difference was not statistically significant (p=0.159). |
Table 1.
Summary of Gastric Lymphoepithelioma-like Carcinomas (n=18)
| Patient (No.) | Age (yr) | Sex | Size (cm)a | Gross | Stage | Location | Lymphovascular invasion | Follow up (month)b |
|---|---|---|---|---|---|---|---|---|
| 1 | 49 | Male | 4.5 | Borrmann 3 | T2N0M0, IB | Fundus | (+) | 81.2 |
| 2 | 58 | Male | 2.5 | Borrmann 1 | T2N0M0, IB | Body | (+) | (−)c |
| 3 | 69 | Female | 5.0 | Borrmann 1 | T4N1M0, IIIA | Body | (+) | 14.5 |
| 4 | 67 | Male | 2.5 | Type IIc | T1N0M0, IA | Body | (−) | 67.4 |
| 5 | 47 | Male | 2.0 | Borrmann 3 | T2N2M0, IIB | Body | (+) | 76.6 |
| 6 | 47 | Male | 2.5 | Borrmann 3 | T2N0M0, IB | Body | (+) | 59.9 |
| 7 | 80 | Male | 3.5 | Borrmann 1 | T2N0M0, IB | Body | (+) | 46.6 |
| 8 | 53 | Male | 1.5 | Type IIc | T1N0M0, IA | Body | (+) | 38.0 |
| 9 | 62 | Female | 1.3 | Type IIc | T1N1M0, IB | Body | (+) | 39.6 |
| 10 | 55 | Male | 6.9 | Borrmann 3 | T4N2M0, IIIB | Antrum | (+) | 10.3 |
| 11 | 52 | Female | 2.7 | Type III | T1N0M0, IA | Body | (+) | 35.4 |
| 12 | 57 | Male | 2.4 | Borrmann 3 | T4N2M1, IV | Body | (+) | 27.6 |
| 13 | 49 | Male | 0.3 | Type III | T1N0M0, IA | Body | (−) | 30.6 |
| 14 | 61 | Male | 4.0 | Borrmann 3 | T2N0M0, IB | Antrum | (+) | 27.2 |
| 15 | 47 | Male | 3.9 | Borrmann 3 | T4N1M0, IIIA | Body | (+) | 15.9 |
| 16 | 50 | Male | 4.0 | Borrmann 3 | T4N1M0, IIIA | Body | (+) | 21.0 |
| 17 | 55 | Male | 5.3 | Borrmann 3 | T3N0M0, IIA | Body | (−) | 6.4 |
| 18 | 51 | Male | 2.1 | Borrmann 3 | T2N1M0, IIA | Cardia | (+) | 19.7 |
Table 2.
Characteristics of Gastric Lymphoepithelioma‐ like Carcinoma (Group A) and Poorly Differentiated Gastric Adenocarcinoma (Group B)
| Group Aa (n=17) | Group B (n=36) | p‐ value | |
|---|---|---|---|
| Sex | 0.237 | ||
| Male | 14 (82.4) | 24 (66.7) | |
| Female | 3 (17.6) | 12 (33.3) | |
| Age (yr) | 55.9 (47–80) | 57.6 (33–74) | 0.547 |
| T stage | 0.888 | ||
| pT1 | 5 (29.4) | 12 (33.3) | |
| pT2 | 6 (35.2) | 9 (25) | |
| pT3 | 1 (5.8) | 3 (8.3) | |
| pT4 | 5 (29.4) | 12 (33.3) | |
| N stage | 0.005 | ||
| pN0 | 9 (52.9) | 20 (55.5) | |
| pN1 | 5 (29.4) | 1 (2.7) | |
| pN2 | 3 (17.6) | 4 (11.1) | |
| pN3 | 0 (0) | 11 (30.5) | |
| M stage | 0.753 | ||
| M0 | 16 (94.1) | 33 (91.7) | |
| M1 | 1 (5.9) | 3 (8.3) | |
| TNM stage | 0.758 | ||
| I | 9 (52.9) | 19 (52.8) | |
| II | 3 (17.6) | 3 (8.3) | |
| III | 4 (23.5) | 11 (30.6) | |
| IV | 1 (5.9) | 3 (8.3) | |
| Borrmann typeb | 0.097 | ||
| Type 1 | 2 (16.7) | 1 (4.2) | |
| Type 2 | 0 (0) | 6 (25) | |
| Type 3 | 10 (83.3) | 17 (70.8) | |
| Type 4 | 0 (0) | 0 (0) | |
| EGC type | 0.059 | ||
| Type I | 0 (0) | 0 (0) | |
| Type IIa | 0 (0) | 0 (0) | |
| Type IIb | 0 (0) | 1 (8.3) | |
| Type IIc | 3 (60) | 11 (91.7) | |
| Type III | 2 (40) | 0 (0) | |
| Locationc | 0.944 | ||
| Distal | 15 (88.2) | 32 (88.9) | |
| Proximal | 2 (11.8) | 4 (11.1) | |
| Size (cm)d | 3.20±1.66 | 4.17±2.78 | 0.19 |
| Lymphovascular i | invasion | 0.424 | |
| (−) | 3 (17.7) | 10 (27.8) | |
| (+) | 14 (82.3) | 26 (72.2) | |
| Treatment | 0.27 | ||
| Resection only | 13 (76.5) | 22 (61.1) | |
| Postop. adj. C | Tx. 4 (23.5) | 14 (38.9) |
Values are presented as mean (range), n (%), or mean±SD. Postop. adj. CTx, postoperative adjuvant chemotherapy.
a In gastric lymphoepithelioma-like carcinoma, 1 person was lost to follow up and was therefore not included in the analysis.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download