Abstract
Background/Aims
Early colon cancer can be effectively diagnosed and treated by colonoscopy, and surveillance colonoscopy is necessary to detect precursor lesions or new early colon cancer. We analyzed the surveillance results of patients with endoscopically resected early colon cancer to evaluate the detection rate of advanced neoplasia and its associated factors.
Methods
We conducted a retrospective study at Soonchunhyang University Seoul Hospital, from May 2003 to December 2011. Patients who underwent endoscopic resection for early colon cancer, showed mucosal and submucosal invasion on histopathologic examination, and received surveillance colonoscopy at least once were enrolled in the current study. Patients who underwent operation and those who were lost during surveillance period were excluded.
Results
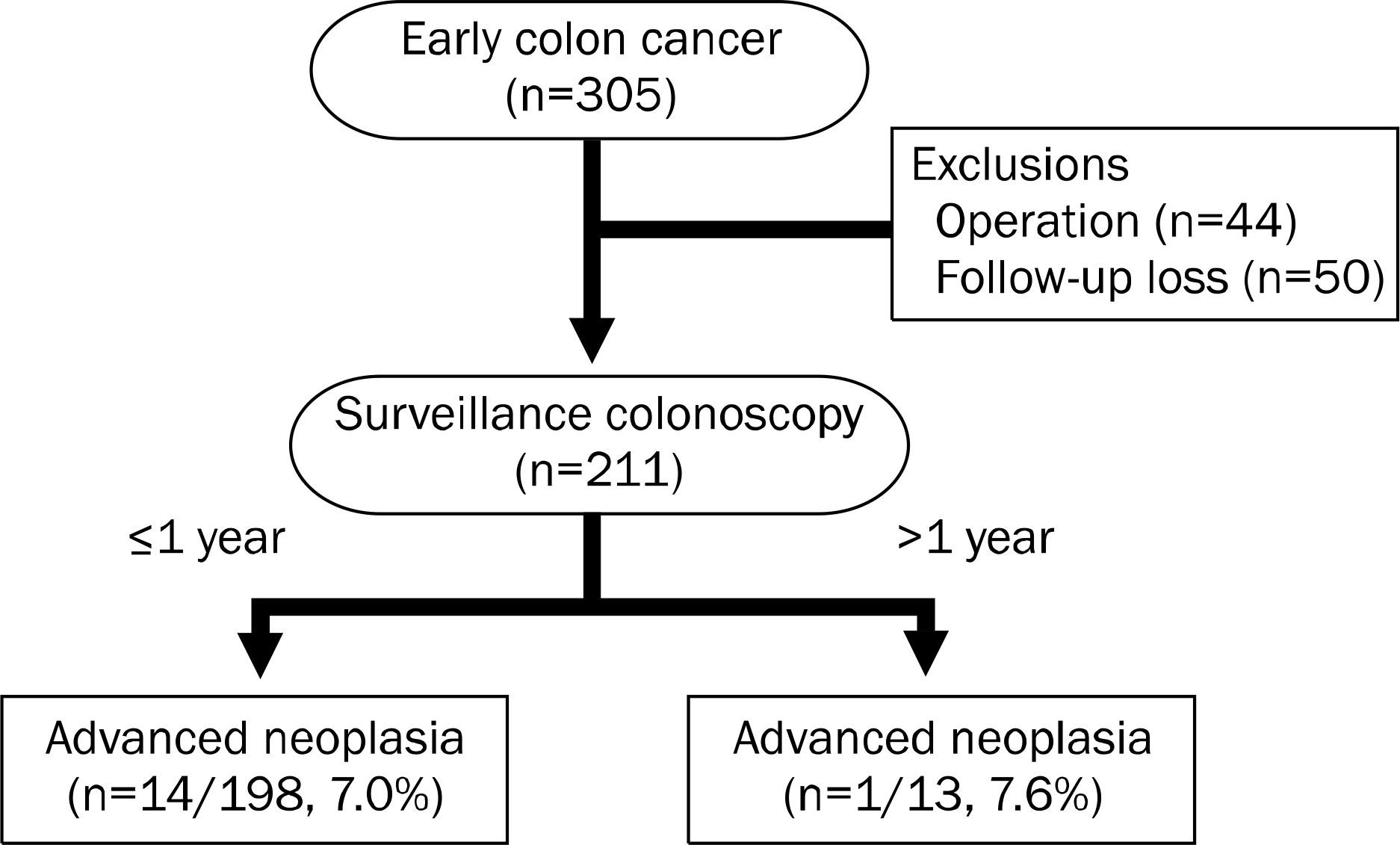
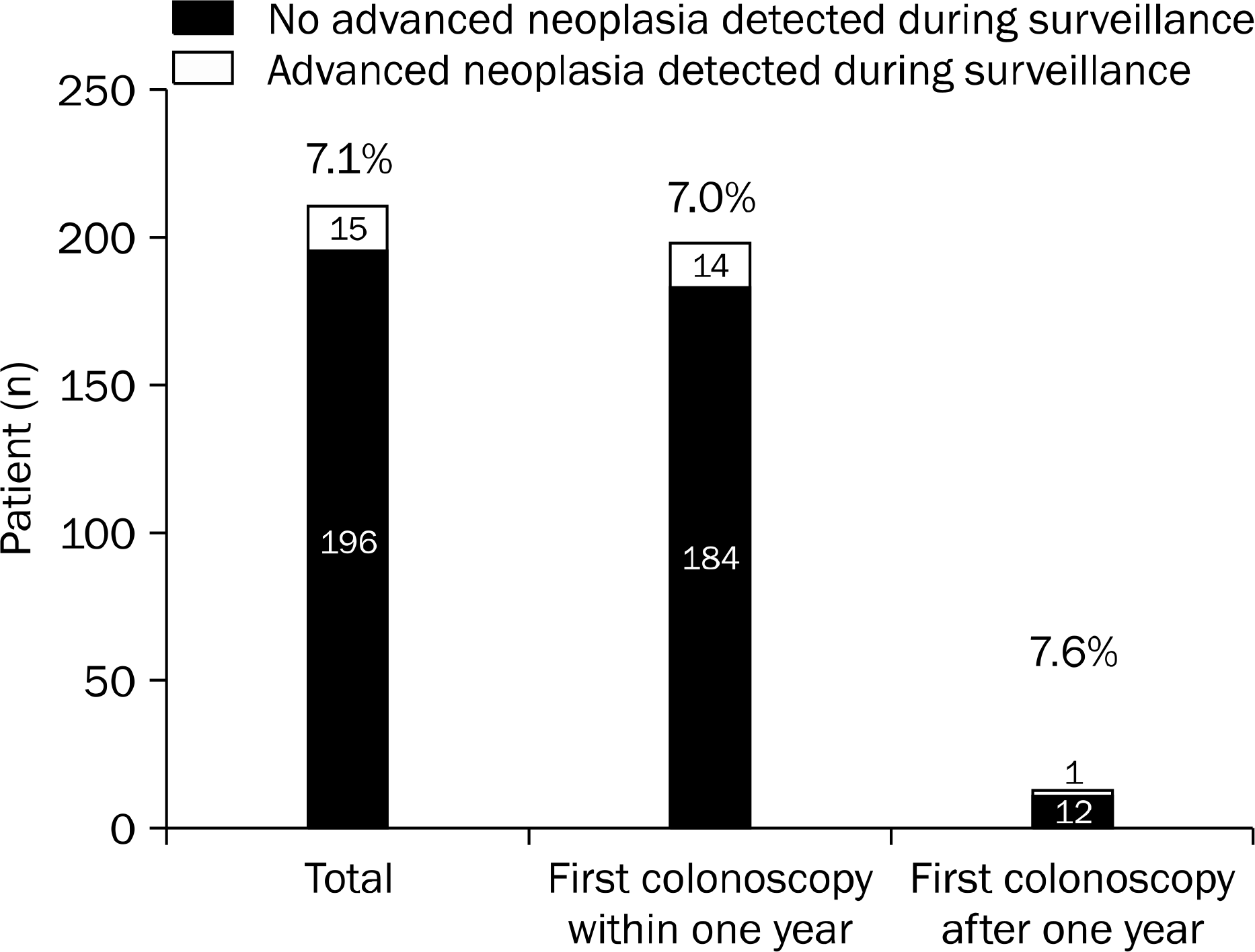
Among a total of 305 patients diagnosed with early colon cancer, 211 patients met our inclusion criteria. Of these patients, 15 (7.1%) advanced neoplasias were detected at first colonoscopy. One hundred ninety-eight patients (93.8%) underwent surveillance colonoscopy within one year and 14 (7.0%) advanced neoplasias were detected in this group of patients. When patients with and without advanced neoplasia at first surveillance colonoscopy performed within one year were compared, inadequate bowel preparation (OR, 18.237; 95% CI, 3.741–88.895; p<0.001) and three or more colon polyps (OR, 9.479; 95% CI, 1.103–81.452; p=0.040) were significant risk factors for detecting advanced neoplasia.
Conclusions
Considering the high detection rate of advanced neoplasia at first surveillance colonoscopy in patients with endoscopically resected early colon cancer, surveillance interval should be within one year, especially when the bowel preparation has been inadequate and three or more colon polyps have been detected.
Go to : 
References
1. Jung KW, Park S, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality and survival in 2006–2007. J Korean Med Sci. 2010; 25:1113–1121.

2. Ministry of Health & Welfare. Annual report of cancer statistics in Korea. Seoul: Ministry of Health & Welfare;2009.
3. Morson B. President's address. The polyp-cancer sequence in the large bowel. Proc R Soc Med. 1974; 67:451–457.
4. Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012; 366:687–696.

5. Citarda F, Tomaselli G, Capocaccia R, Barcherini S, Crespi M. Italian Multicentre Study Group. Efficacy in standard clinical practice of colonoscopic polypectomy in reducing colorectal cancer incidence. Gut. 2001; 48:812–815.

6. Loeve F, van Ballegooijen M, Snel P, Habbema JD. Colorectal cancer risk after colonoscopic polypectomy: a population-based study and literature search. Eur J Cancer. 2005; 41:416–422.

7. Lund JN, Scholefield JH, Grainge MJ, et al. Risks, costs, and compliance limit colorectal adenoma surveillance: lessons from a randomised trial. Gut. 2001; 49:91–96.

8. Winawer SJ, Zauber AG, O'Brien MJ, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N Engl J Med. 1993; 328:901–906.
9. Hong SN, Yang DH, Kim YH, et al. MultiSociety Task Force for-Development of Guidelines for Colorectal Polyp Screening, Surveillance and Management. Korean guidelines for post-polypectomy colonoscopic surveillance. Korean J Gastroenterol. 2012; 59:99–117.

10. Lee HJ, Jeong HY, Park NH, et al. Follow-up results of endoscopic mucosal resection for early colorectal cancer. Korean J Gastroenterol. 2011; 57:230–236.

11. Kang JG, Kim DH, Kim CH, Lee SH, Choi YJ. Clinical characteristics of synchronous multiple colorectal cancer. J Koeran Soc Coloproctol. 2006; 22:418–423.
12. Jung SH, Kim HC, Kim AY, et al. Colorectal cancer presenting as an early recurrence within 1 year after a curative resection. J Korean Soc Coloproctol. 2008; 24:265–272.

13. Kikuchi R, Takano M, Takagi K, et al. Management of early invasive colorectal cancer. Risk of recurrence and clinical guidelines. Dis Colon Rectum. 1995; 38:1286–1295.
14. Carlsson G, Petrelli NJ, Nava H, Herrera L, Mittelman A. The value of colonoscopic surveillance after curative resection for colorectal cancer or synchronous adenomatous polyps. Arch Surg. 1987; 122:1261–1263.

15. Atkin WS, Saunders BP. British Society for Gastroenterology; Association of Coloproctology for Great Britain and Ireland. Surveillance guidelines after removal of colorectal adenomatous polyps. Gut. 2002; 51(Suppl 5):V6–V9.

16. Kim HU, Kim YH, Song SY. Characteristics of early colon cancer in Korea. Korean J Gastrointest Endosc. 2004; 29:126–132.
17. Kudo S, Kashida H, Tamura T, et al. Colonoscopic diagnosis and management of nonpolypoid early colorectal cancer. World J Surg. 2000; 24:1081–1090.

18. Chung SJ, Kim YS, Yang SY, et al. Five-year risk for advanced colorectal neoplasia after initial colonoscopy according to the baseline risk stratification: a prospective study in 2452 asymptomatic Koreans. Gut. 2011; 60:1537–1543.

19. Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009; 69(3 Pt 2):620–625.

20. Tanimoto T, Tanaka S, Haruma K, et al. Growth patterns in various macroscopic types of noninvasive intramucosal colorectal carcinoma with special reference to apoptosis and cell proliferation. Dis Colon Rectum. 1998; 41:1376–1384.

21. Tanaka S, Oka S, Chayama K. Advances of an internal treatment for early colorectal carcinoma. Nihon Shokakibyo Gakkai Zasshi. 2004; 101:486–494.
22. Winawer SJ, Zauber AG, Fletcher RH, et al. US MultiSociety Task Force on Colorectal Cancer; American Cancer Society. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US MultiSociety Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology. 2006; 130:1872–1885.

23. Arditi C, Gonvers JJ, Burnand B, et al. EPAGE II Study Group. Appropriateness of colonoscopy in Europe (EPAGE II). Surveillance after polypectomy and after resection of colorectal cancer. Endoscopy. 2009; 41:209–217.
24. Hong SN, Yang DH, Kim YH, et al. MultiSociety Task Force for-Development of Guidelines for Colorectal Polyp Screening, Surveillance and Management. Korean guidelines for post-polypectomy colonoscopic surveillance. Korean J Gastroenterol. 2012; 59:99–117.

25. Burt RW, Barthel JS, Dunn KB, et al. NCCN. NCCN clinical practice guidelines in oncology. Colorectal cancer screening. J Natl Compr Canc Netw. 2010; 8:8–61.
26. Lee BI, Hong SP, Kim SE, et al. MultiSociety Task Force for Development of Guidelines for Colorectal Polyp Screening, Surveillance and Management. Korean guidelines for colorectal cancer screening and polyp detection. Korean J Gastroenterol. 2012; 59:65–84.

27. Cottet V, Jooste V, Fournel I, Bouvier AM, Faivre J, Bonithon-Kopp C. Longterm risk of colorectal cancer after adenoma removal: a population-based cohort study. Gut. 2012; 61:1180–1186.

Go to : 
Table 1.
Comparison of Initial Endoscopic and Histologic Characteristics between Group A and B
| Characteristic | Total (n=211) | Group A (n=14) | Group B (n=197) | p-value |
|---|---|---|---|---|
| Age (yr) | 61.1±9.8 | 62.2±9.5 | 61.1±9.9 | 0.667 |
| Sex (male) | 149 (70.6) | 9 (64.3) | 140 (71.1) | 0.558 |
| Location | ||||
| Right | 57 (27.0) | 3 (21.4) | 54 (27.4) | 0.763 |
| Left | 154 (72.9) | 11 (78.6) | 143 (72.6) | |
| Size (mm) | ||||
| <10 | 39 (18.5) | 3 (21.4) | 36 (18.3) | 0.726 |
| ≥10 | 172 (81.5) | 11 (78.6) | 161 (81.7) | |
| Morphology | ||||
| Polypoid | 157 (74.4) | 12 (85.7) | 145 (73.6) | 0.526 |
| Non-polypoid | 54 (25.6) | 2 (14.3) | 52 (26.4) | |
| Number of polyps | ||||
| <3 | 84 (39.8) | 1 (7.1) | 83 (42.1) | 0.010a |
| ≥3 | 127 (60.2) | 13 (92.9) | 114 (57.9) | |
| BBPS | ||||
| <6 | 19 (9.0) | 6 (42.9) | 13 (6.6) | <0.001a |
| ≥6 | 192 (90.9) | 8 (57.1) | 184 (93.4) | |
| LVI | ||||
| No | 210 (99.5) | 14 (100) | 196 (99.5) | >0.999 |
| Yes | 1 (0.5) | 0 (0) | 1 (0.5) | |
| Resection margin | ||||
| Negative | 197 (93.4) | 14 (100) | 183 (92.9) | 0.605 |
| Positive | 14 (6.6) | 0 (0) | 14 (7.1) | |
| Death | ||||
| Mucosa | 179 (84.8) | 12 (85.7) | 167 (84.8) | >0.999 |
| SM1 | 32 (15.2) | 2 (14.3) | 30 (15.2) |
Table 2.
Multivariate Analysis of Independent Factors for Detecting Advanced Neoplasia at First Surveillance Colonoscopy within One Year
| Category | Univariate analysis (p-value) |
Multivariate analysis |
|
|---|---|---|---|
| OR (95% CI) | p-valuea | ||
| Site | 0.763 | NA | NA |
| Morphology | 0.526 | NA | NA |
| Polyp size (mm) | 0.726 | NA | NA |
| Total polyps | 0.010 | 9.479 | 0.040 |
| (≥3 polyps) | (1.103–81.452) | ||
| Poor bowel | <0.001 | 18.237 | <0.001 |
| preparation | (3.741–88.895) | ||




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download