Abstract
Occult HBV infection (OBI) is defined as presence of HBV DNA in the liver tissue in patients with serologically undetectable HBsAg. There are differences in virologic and serological profiles of OBI. Majority of OBI are positive for anti-HBs and/or anti-HBc and minor portion are negative for all HBV markers. However, there are no HBV mutations in the surface and its regulatory regions. HBV infection persists by the presence of covalently closed circular DNA (cccDNA) within the infected hepatocytes, which serves as a reservoir for future infection. OBI increases the risk of HBV transmission through transfusion, hemodialysis, and organ transplantation. Therefore effective measures should be employed to screen OBI. Antiviral therapy is needed in HBsAg-negative transplant patients who are anti-HBc positive to prevent the recurrence of HBV infection. Since HBV replication is strongly suppressed by immune surveillance system in OBI patients, immunosuppression results in massive HBV replication. This leads to acute hepatitis and sometimes mortality when immune surveillance is recovered after stopping immunosuppressive drugs/anticancer chemotherapy. Therefore, narrow surveillance is required to recognize the viral reactivation and start antiviral agents during immunosuppressive therapy/anticancer chemotherapy in patients with OBI.
Go to : 
References
1. Raimondo G, Allain JP, Brunetto MR, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol. 2008; 49:652–657.

2. Conjeevaram HS, Lok AS. Occult hepatitis B virus infection: a hidden menace? Hepatology. 2001; 34:204–206.

3. Mulrooney-Cousins PM, Michalak TI. Persistent occult hepatitis B virus infection: experimental findings and clinical implications. World J Gastroenterol. 2007; 13:5682–5686.

4. Chu CJ, Lee SD. Occult hepatitis B virus infection in patients with chronic hepatitis C: An actor behind the scene or just a by-stander? J Gastroenterol Hepatol. 2010; 25:221–223.

6. Regan FA, Hewitt P, Barbara JA, Contreras M. Prospective investigation of transfusion transmitted infection in recipients of over 20,000 units of blood. TTI Study Group. BMJ. 2000; 320:403–406.
7. Saraswat S, Banerjee K, Chaudhury N, et al. Post-transfusion hepatitis type B following multiple transfusions of HBsAg-negative blood. J Hepatol. 1996; 25:639–643.

8. Raimondo G, Caccamo G, Filomia R, Pollicino T. Occult HBV infection. Semin Immunopathol. 2013; 35:39–52.

9. Said ZN. An overview of occult hepatitis B virus infection. World J Gastroenterol. 2011; 17:1927–1938.

10. Allain JP. Occult hepatitis B virus infection: implications in transfusion. Vox Sang. 2004; 86:83–91.

11. Satake M, Taira R, Yugi H, et al. Infectivity of blood components with low hepatitis B virus DNA levels identified in a lookback program. Transfusion. 2007; 47:1197–1205.
12. Taira R, Satake M, Momose S, et al. Residual risk of transfusion-transmitted hepatitis B virus (HBV) infection caused by blood components derived from donors with occult HBV infection in Japan. Transfusion. 2013; 53:1393–1404.

13. Seo DH, Whang DH, Song EY, Kim HS, Park Q. Prevalence of antibodies to hepatitis B core antigen and occult hepatitis B virus infections in Korean blood donors. Transfusion. 2011; 51:1840–1846.

14. Samuel D, Forns X, Berenguer M, et al. Report of the mono-thematic EASL conference on liver transplantation for viral hepatitis (Paris, France, January 12–14, 2006). J Hepatol. 2006; 45:127–143.
15. Cholongitas E, Papatheodoridis GV, Burroughs AK. Liver grafts from anti-hepatitis B core positive donors: a systematic review. J Hepatol. 2010; 52:272–279.

16. De Feo TM, Poli F, Mozzi F, Moretti MP, Scalamogna M. Collaborative Kidney, Liver and Heart North Italy Transplant Program Study Groups. Risk of transmission of hepatitis B virus from anti-HBC positive cadaveric organ donors: a collaborative study. Transplant Proc. 2005; 37:1238–1239.

17. Gwak GY, Huh W, Lee DH, et al. Occult hepatitis B virus infection in chronic hemodialysis patients in Korea. Hepatogastroenterology. 2008; 55:1721–1724.
18. Yoo JH, Hwang SG, Yang DH, et al. Prevalence of occult hepatitis B virus infection in hemodialysis patients. Korean J Gastroenterol. 2013; 61:209–214.

19. Minuk GY, Sun DF, Greenberg R, et al. Occult hepatitis B virus infection in a North American adult hemodialysis patient population. Hepatology. 2004; 40:1072–1077.

20. Kwon CI, Hwang SG, Shin SJ, et al. Occult hepatitis B virus infection in pregnant woman and its clinical implication. Liver Int. 2008; 28:667–674.

21. Zerbini A, Pilli M, Boni C, et al. The characteristics of the cellmediated immune response identify different profiles of occult hepatitis B virus infection. Gastroenterology. 2008; 134:1470–1481.

22. Bes M, Vargas V, Piron M, et al. T cell responses and viral variability in blood donation candidates with occult hepatitis B infection. J Hepatol. 2012; 56:765–774.

23. Galbraith RM, Eddleston AL, Williams R, Zuckerman AJ. Fulminant hepatic failure in leukaemia and choriocarcinoma related to withdrawal of cytotoxic drug therapy. Lancet. 1975; 2:528–530.

24. Lalazar G, Rund D, Shouval D. Screening, prevention and treatment of viral hepatitis B reactivation in patients with haematological malignancies. Br J Haematol. 2007; 136:699–712.

25. Schmeltzer P, Sherman KE. Occult hepatitis B: clinical implications and treatment decisions. Dig Dis Sci. 2010; 55:3328–3335.

26. Kusumoto S, Tanaka Y, Ueda R, Mizokami M. Reactivation of hepatitis B virus following rituximab-plus-steroid combination chemotherapy. J Gastroenterol. 2011; 46:9–16.

27. Zachou K, Sarantopoulos A, Gatselis NK, et al. Hepatitis B virus reactivation in hepatitis B virus surface antigen negative patients receiving immunosuppression: A hidden threat. World J Hepatol. 2013; 5:387–392.

28. Kim EB, Kim DS, Park SJ, Park Y, Rho KH, Kim SJ. Hepatitis B virus reactivation in a surface antigen-negative and antibody-positive patient after rituximab plus CHOP chemotherapy. Cancer Res Treat. 2008; 40:36–38.

29. Chung SM, Sohn JH, Kim TY, et al. Fulminant hepatic failure with hepatitis B virus reactivation after rituximab treatment in a patient with resolved hepatitis B. Korean J Gastroenterol. 2010; 55:266–269.

30. Ha SH, Park YM, Hong SP, et al. De novo superinfection of hepatitis B virus in an anti-HBs positive patient with recurrent hepatitis C following liver transplantation. Gut Liver. 2011; 5:248–252.

31. Jung HM, Jun DW, Min JY, et al. A case of acute hepatitis B by occult HBV infection without HbsAg seroconversion. Korean J Med. 2012; 83:619–623.

32. Shouval D, Shibolet O. Immunosuppression and HBV reactivation. Semin Liver Dis. 2013; 33:167–177.

33. Korean Association for the Study of the Liver. KASL Clinical Practice Guidelines: Management of chronic hepatitis B. Clin Mol Hepatol. 2012; 18:109–162.
34. Hui CK, Cheung WW, Zhang HY, et al. Kinetics and risk of de novo hepatitis B infection in HBsAg-negative patients undergoing cytotoxic chemotherapy. Gastroenterology. 2006; 131:59–68.

35. Barclay S, Pol S, Mutimer D, et al. The management of chronic hepatitis B in the immunocompromised patient: recommendations from a single topic meeting. J Clin Virol. 2008; 41:243–254.

Go to : 
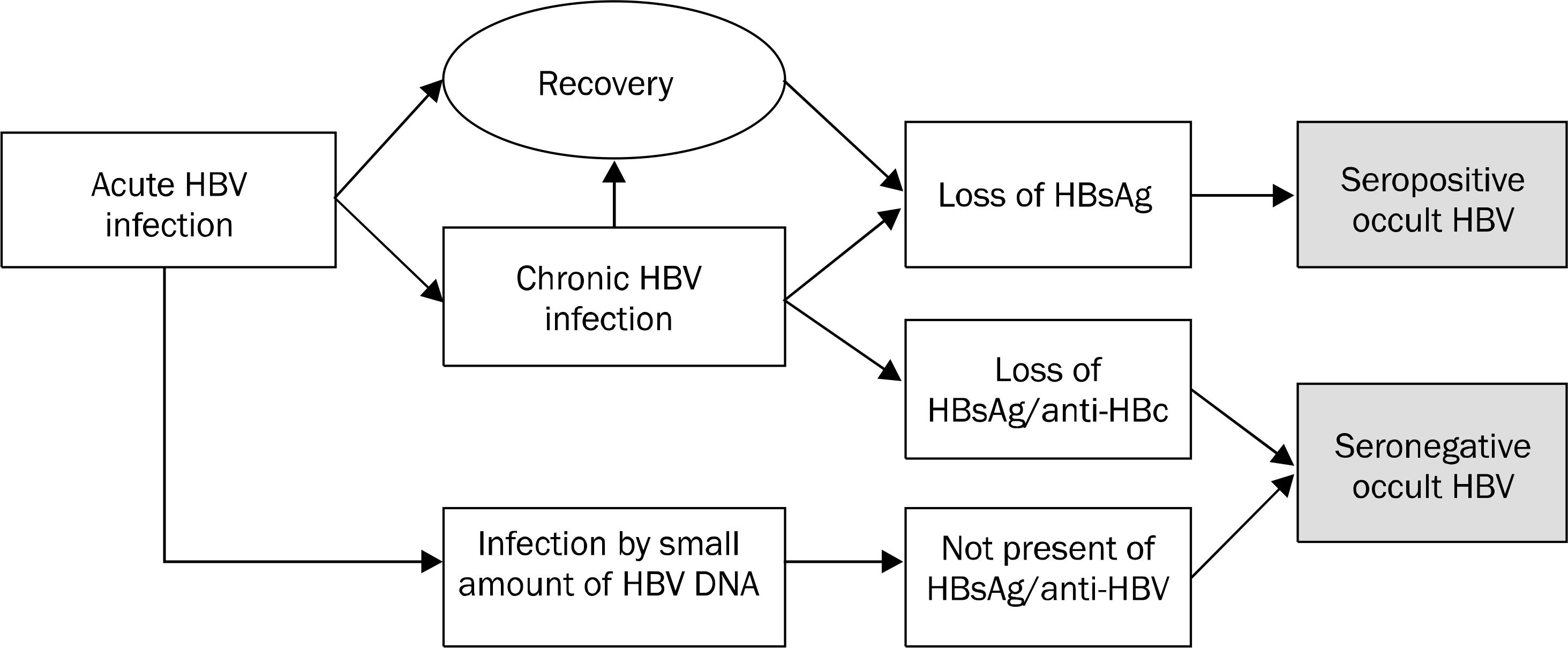 | Fig. 1.Seropositive and seronegative occult HBV infection. Majority of occult HBV infections are positive for anti-HBs and/or anti-HBc and minor portion are negative for all HBV markers. |
Table 1.
Reactivation of Occult HBV Reported in Korea




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download