Abstract
Background/Aims
Although general guidelines have suggested weight-based dosing of azathioprine (AZA, 2.5 mg/kg/day) for Crohn's disease (CD), a substantial number of patients develop bone marrow suppression. The aim of this study was to evaluate the maximum dose of AZA not based on weight but titrated according to the lower limit of leukocyte count for maintaining remission in patients with CD.
Methods
Among a total of seventy-eight patients with CD, who had been followed-up at Kosin University Gospel Hospital (Busan, Korea) from 2010 to 2011, those treated with the maximum dose of AZA meeting both drug-tolerability and leukocytes count of more than 4,000/mm3 for steroid-free maintaining remission were enrolled. The titrated maximum AZA dose and its relationship with weight were evaluated.
Results
A total of 42 patients (male, 32 patients; mean age, 31 years) were enrolled. The maximum dose of AZA was 49.1 mg/day. The dose per weight was 0.87 mg/kg/day and negatively correlated with body weight (γ=−0.51, p=0.01) and BMI (γ=−0.33, p=0.034). AZA dose per weight in the below 40 years old group was significantly higher than that in the above 40 years old group (p=0.039).
Conclusions
Dose decision of AZA based only on weight could put the patients to inappropriately low or high dose resulting in need of additional therapy or serious side effect, respectively. Therefore, the maximum dose-titration based on the lower limit of leukocyte count and tolerability is a novel and a valuable strategy in deciding the dose of thiopurines.
Go to : 
References
1. Lennard L. The clinical pharmacology of 6-mercaptopurine. Eur J Clin Pharmacol. 1992; 43:329–339.

3. Candy S, Wright J, Gerber M, Adams G, Gerig M, Goodman R. A controlled double blind study of azathioprine in the management of Crohn's disease. Gut. 1995; 37:674–678.

4. Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut. 2002; 50:485–489.

5. Sandborn W, Sutherland L, Pearson D, May G, Modigliani R, Prantera C. Azathioprine or 6-mercaptopurine for inducing remission of Crohn's disease. Cochrane Database Syst Rev. 2000; (2):CD000545.
6. Kim WH, Cho JH, Kim TI. Rational dosing of azathioprine and 6-mercaptopurine in inflammatory bowel diseases. Korean J Gastroenterol. 2003; 41:423–437.
7. Su C, Lichtenstein GR. Treatment of inflammatory bowel disease with azathioprine and 6-mercaptopurine. Gastroenterol Clin North Am. 2004; 33:209–234.

8. Present DH, Meltzer SJ, Krumholz MP, Wolke A, Korelitz BI. 6-Mercaptopurine in the management of inflammatory bowel disease: short- and long-term toxicity. Ann Intern Med. 1989; 111:641–649.

9. Sandborn WJ, Tremaine WJ, Wolf DC, et al. Lack of effect of intravenous administration on time to respond to azathioprine for steroid-treated Crohn's disease. North American Azathioprine Study Group. Gastroenterology. 1999; 117:527–535.
10. Barabino A, Torrente F, Ventura A, Cucchiara S, Castro M, Barbera C. Azathioprine in paediatric inflammatory bowel disease: an Italian multicentre survey. Aliment Pharmacol Ther. 2002; 16:1125–1130.

11. Fraser AG, Orchard T, Jewell DP. Side effects of azathioprine treatment given for inflammatory bowel disease: a 30 year audit. Gastroenterology. 2000; 118(A4):201.
12. Lee HJ, Yang SK, Kim KJ, et al. The safety and efficacy of azathioprine and 6-mercaptopurine in the treatment of Korean patients with crohn's disease. Intest Res. 2009; 7:22–31.
13. Hyun KH, Lee SH, Shin JM, et al. Frequency of bone marrow toxicity by using pattern of azathioprine in inflammatory bowel disease patients. Intest Res. 2012; 10:244–250.

14. McLeod HL, Pritchard SC, Githang'a J, et al. Ethnic differences in thiopurine methyltransferase pharmacogenetics: evidence for allele specificity in Caucasian and Kenyan individuals. Pharmacogenetics. 1999; 9:773–776.
15. Lichtenstein GR. Use of laboratory testing to guide 6-mercaptopur-ine/azathioprine therapy. Gastroenterology. 2004; 127:1558–1564.

16. Andoh A, Tsujikawa T, Ban H, et al. Monitoring 6-thioguanine nucleotide concentrations in Japanese patients with inflammatory bowel disease. J Gastroenterol Hepatol. 2008; 23:1373–1377.

17. Goldenberg BA, Rawsthorne P, Bernstein CN. The utility of 6-thio-guanine metabolite levels in managing patients with inflammatory bowel disease. Am J Gastroenterol. 2004; 99:1744–1748.

18. Gupta P, Gokhale R, Kirschner BS. 6-mercaptopurine metabolite levels in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2001; 33:450–454.
19. Osterman MT, Kundu R, Lichtenstein GR, Lewis JD. Association of 6-thioguanine nucleotide levels and inflammatory bowel disease activity: a metaanalysis. Gastroenterology. 2006; 130:1047–1053.

21. Hibi T, Naganuma M, Kitahora T, Kinjyo F, Shimoyama T. Lowdose azathioprine is effective and safe for maintenance of remission in patients with ulcerative colitis. J Gastroenterol. 2003; 38:740–746.

22. Hibi T, Inoue N, Ogata H, Naganuma M. Introduction and overview: recent advances in the immunotherapy of inflammatory bowel disease. J Gastroenterol. 2003; 38(Suppl 15):36–42.
23. Kim PS, Zlatanic J, Korelitz BI, Gleim GW. Optimum duration of treatment with 6-mercaptopurine for Crohn's disease. Am J Gastroenterol. 1999; 94:3254–3257.

24. Sahmoud T, Hoctin-Boes G, Modigliani R, et al. Identifying patients with a high risk of relapse in quiescent Crohn's disease. The GETAID Group. The Groupe d'Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gut. 1995; 37:811–818.
25. Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003; 3:521–533.

26. Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005; 307:1920–1925.

27. Ginaldi L, De Martinis M, D'Ostilio A, et al. The immune system in the elderly: II. Specific cellular immunity. Immunol Res. 1999; 20:109–115.
Go to : 
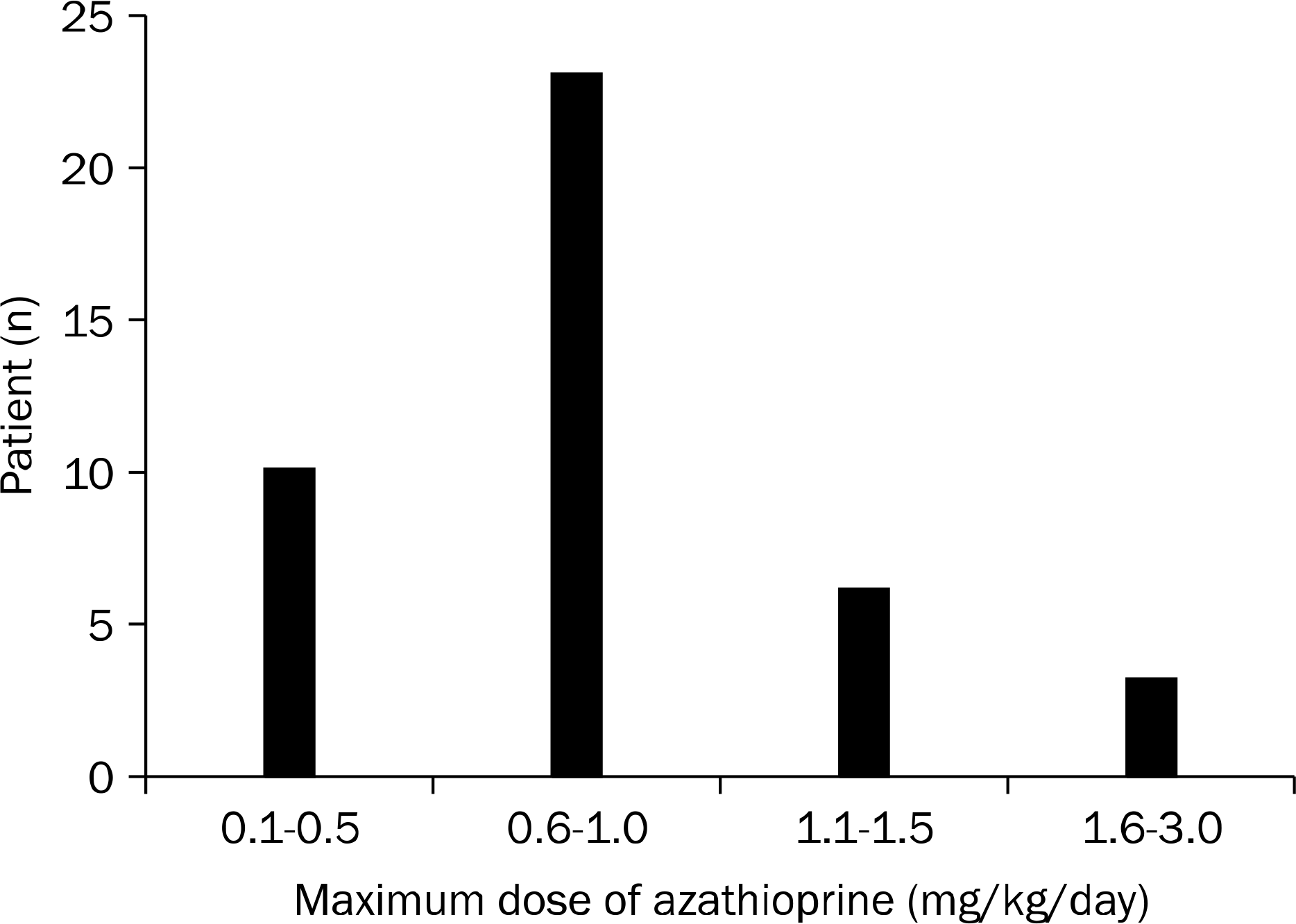 | Fig. 1.Distribution of maximal dose of azathioprine. The number of patients according to the azathioprine dose/body weight/day were 10 (23.8%) in 0.1–0.5 mg/kg/day group, 23 (54.8%) in 0.6–1.0 mg/kg/day group, 6 (14.3%) in 1.1–1.5 mg/kg/day group, and 3 (7.2%) in 1.6–3.0 mg/kg/day group. |
Table 1.
Clinical Characteristics of the Patients
Table 2.
Correlation between Physical Measurements and Azathioprine Dose
| Pearson's correlation (γ) | p-value | |
|---|---|---|
| Azathioprine dose/body weight versus weight | −0.51 | 0.010 |
| Azathioprine dose/weight versus body mass index | −0.33 | 0.034 |
Table 3.
Comparison of Azathioprine Dose according to Clinical Factors




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download