Abstract
One of the most critical characteristics of colorectal cancer (CRC) is the difference between proximal (right-sided colon cancer, RCC) and distal (left-sided colon cancer, LCC) disease. The recent CRC studies showed the unique characteristics of RCC; RCCs were more prevalent in women than men and old patients, and the age difference between RCC and LCC was more apparent in women. Moreover, relatively poor protection against RCC by colonoscopy is a clearly hot issue for alarm. Thus, the left and right colon have been considered as dichotomous or even different organs in the view of molecular, histopathological, epidemiologic and clinical bases for over three decades. However, the evolutionary data suggesting linearity from the rectum to ascending colon beyond the simple right-left dichotomization in the views of cancer molecular features and site-specific clinicopathological differences, support the need for a paradigm shift to the colorectal continuum model rather than the traditional two-colon concept. This new multi-segmental or colorectal continuum hypothesis would provide both the better understanding of the complex etiology of colorectal carcinogenesis and the tailored preventive and therapeutic strategies for CRC including individualized CRC screening programs.
Go to : 
References
1. Ogino S, Galon J, Fuchs CS, Dranoff G. Cancer immunol-ogy–analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol. 2011; 8:711–719.

2. Potter J, Hunter D. Colorectal cancer: epidemiology. Potter J, Lindor N, editors. Genetics of colorectal cancer. New York: Springer;2009. p. 5–25.

3. McMichael AJ, Potter JD. Reproduction, endogenous and exogenous sex hormones, and colon cancer: a review and hypothesis. J Natl Cancer Inst. 1980; 65:1201–1207.
4. Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, et al. Women's Health Initiative Investigators. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004; 350:991–1004.

5. McMichael AJ, Potter JD. Host factors in carcinogenesis: certain bile-acid metabolic profiles that selectively increase the risk of proximal colon cancer. J Natl Cancer Inst. 1985; 75:185–191.
6. Jass J. Pathway and pathology. Potter J, Lindor N, editors. Genetics of colorectal cancer. New York: Springer;2009. p. 97–121.
7. Jensen OM. Different age and sex relationship for cancer of subsites of the large bowel. Br J Cancer. 1984; 50:825–829.

8. Benedix F, Kube R, Meyer F, Schmidt U, Gastinger I, Lippert H. Colon/Rectum Carcinomas (Primary Tumor) Study Group. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum. 2010; 53:57–64.

9. Siegel RL, Ward EM, Jemal A. Trends in colorectal cancer incidence rates in the United States by tumor location and stage, 1992–2008. Cancer Epidemiol Biomarkers Prev. 2012; 21:411–416.

10. Hutchins G, Southward K, Handley K, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol. 2011; 29:1261–1270.

11. Weiss JM, Pfau PR, O'Connor ES, et al. Mortality by stage for right- versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results–Medicare data. J Clin Oncol. 2011; 29:4401–4409.

13. Gervaz P, Bucher P, Morel P. Two colons-two cancers: paradigm shift and clinical implications. J Surg Oncol. 2004; 88:261–266.

15. Albuquerque C, Bakker ER, van Veelen W, Smits R. Colorectal cancers choosing sides. Biochim Biophys Acta. 2011; 1816:219–231.

16. Yamauchi M, Morikawa T, Kuchiba A, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012; 61:847–854.

17. Beart RW, Melton LJ 3rd, Maruta M, Dockerty MB, Frydenberg HB, O'Fallon WM. Trends in right and left-sided colon cancer. Dis Colon Rectum. 1983; 26:393–398.

18. Butcher D, Hassanein K, Dudgeon M, Rhodes J, Holmes FF. Female gender is a major determinant of changing subsite distribution of colorectal cancer with age. Cancer. 1985; 56:714–716.

19. Delattre O, Olschwang S, Law DJ, et al. Multiple genetic alterations in distal and proximal colorectal cancer. Lancet. 1989; 2:353–356.

20. Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med. 1990; 113:779–788.

21. Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009; 150:1–8.

22. Arain MA, Sawhney M, Sheikh S, et al. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol. 2010; 105:1189–1195.

23. Hetzel JT, Huang CS, Coukos JA, et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol. 2010; 105:2656–2664.

24. Neugut AI, Lebwohl B. Colonoscopy vs sigmoidoscopy screening: getting it right. JAMA. 2010; 304:461–462.
25. Ogino S, Goel A. Molecular classification and correlates in colorectal cancer. J Mol Diagn. 2008; 10:13–27.

26. Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997; 386:623–627.

27. Markowitz SD, Bertagnolli MM. Molecular origins of cancer: molecular basis of colorectal cancer. N Engl J Med. 2009; 361:2449–2460.
28. Pritchard CC, Grady WM. Colorectal cancer molecular biology moves into clinical practice. Gut. 2011; 60:116–129.

29. Curtin K, Slattery ML, Samowitz WS. CpG island methylation in colorectal cancer: past, present and future. Patholog Res Int. 2011; 2011:902674.

30. Lao VV, Grady WM. Epigenetics and colorectal cancer. Nat Rev Gastroenterol Hepatol. 2011; 8:686–700.

31. Wong JJ, Hawkins NJ, Ward RL, Hitchins MP. Methylation of the 3p22 region encompassing MLH1 is representative of the CpG island methylator phenotype in colorectal cancer. Mod Pathol. 2011; 24:396–411.

32. Hughes LA, Khalid-de Bakker CA, Smits KM, et al. The CpG island methylator phenotype in colorectal cancer: progress and problems. Biochim Biophys Acta. 2012; 1825:77–85.

33. Samowitz WS, Albertsen H, Herrick J, et al. Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology. 2005; 129:837–845.

34. Weisenberger DJ, Siegmund KD, Campan M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006; 38:787–793.

35. Nosho K, Irahara N, Shima K, et al. Comprehensive biostatistical analysis of CpG island methylator phenotype in colorectal cancer using a large population-based sample. PLoS One. 2008; 3:e3698.

36. Tanaka N, Huttenhower C, Nosho K, et al. Novel application of structural equation modeling to correlation structure analysis of CpG island methylation in colorectal cancer. Am J Pathol. 2010; 177:2731–2740.

37. Zlobec I, Bihl M, Foerster A, Rufle A, Lugli A. Comprehensive analysis of CpG island methylator phenotype (CIMP)-high, -low, and -negative colorectal cancers based on protein marker expression and molecular features. J Pathol. 2011; 225:336–343.

38. Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012; 487:330–337.
39. Thörn M, Bergström R, Kressner U, Sparén P, Zack M, Ekbom A. Trends in colorectal cancer incidence in Sweden 1959–93 by gender, localization, time period, and birth cohort. Cancer Causes Control. 1998; 9:145–152.
40. Chauvenet M, Cottet V, Lepage C, Jooste V, Faivre J, Bouvier AM. Trends in colorectal cancer incidence: a period and birth-cohort analysis in a well-defined French population. BMC Cancer. 2011; 11:282.

41. Limsui D, Vierkant RA, Tillmans LS, et al. Cigarette smoking and colorectal cancer risk by molecularly defined subtypes. J Natl Cancer Inst. 2010; 102:1012–1022.

42. Larsson SC, Wolk A. Meat consumption and risk of colorectal cancer: a metaanalysis of prospective studies. Int J Cancer. 2006; 119:2657–2664.

43. Rothwell PM, Wilson M, Elwin CE, et al. Longterm effect of aspirin on colorectal cancer incidence and mortality:20-year fol-low-up of five randomised trials. Lancet. 2010; 376:1741–1750.
44. Morimoto LM, Newcomb PA, Ulrich CM, Bostick RM, Lais CJ, Potter JD. Risk factors for hyperplastic and adenomatous polyps: evidence for malignant potential? Cancer Epidemiol Biomarkers Prev. 2002; 11:1012–1018.
45. Slattery ML, Curtin K, Anderson K, et al. Associations between cigarette smoking, lifestyle factors, and microsatellite instability in colon tumors. J Natl Cancer Inst. 2000; 92:1831–1836.

46. LaPointe LC, Dunne R, Brown GS, et al. Map of differential transcript expression in the normal human large intestine. Physiol Genomics. 2008; 33:50–64.

47. Worthley DL, Whitehall VL, Buttenshaw RL, et al. DNA methylation within the normal colorectal mucosa is associated with pathway-specific predisposition to cancer. Oncogene. 2010; 29:1653–1662.

48. Schernhammer ES, Giovannucci E, Kawasaki T, Rosner B, Fuchs CS, Ogino S. Dietary folate, alcohol and B vitamins in relation to LINE-1 hypomethylation in colon cancer. Gut. 2010; 59:794–799.

49. Hughes LA, Simons CC, van den Brandt PA, et al. Body size, physical activity and risk of colorectal cancer with or without the CpG island methylator phenotype (CIMP). PLoS One. 2011; 6:e18571.

50. Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012; 22:292–298.

51. Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012; 22:299–306.

52. Li X, LeBlanc J, Truong A, et al. A metaproteomic approach to study human-microbial ecosystems at the mucosal luminal interface. PLoS One. 2011; 6:e26542.

53. Stearns JC, Lynch MD, Senadheera DB, et al. Bacterial bio-geography of the human digestive tract. Sci Rep. 2011; 1:170.

54. Benedix F, Schmidt U, Mroczkowski P, Gastinger I, Lippert H, Kube R. Study Group "Colon/Rectum Carcinoma (Primary Tumor)". Colon carcinoma–classification into right and left sided cancer or according to colonic subsite?–Analysis of 29,568 patients. Eur J Surg Oncol. 2011; 37:134–139.
55. Catenacci DV, Kozloff M, Kindler HL, Polite B. Personalized colon cancer care in 2010. Semin Oncol. 2011; 38:284–308.

56. Wray CM, Ziogas A, Hinojosa MW, et al. Tumor subsite location within the colon is prognostic for survival after colon cancer diagnosis. Dis Colon Rectum. 2009; 52:1359–1366.

57. Park HJ, Kim HS, Won CS, et al. High level quality colonoscopy identifies unique characteristics of colorectal adenomas in asymptomatic older female. Clin Endosc. 2012; 45:S147–S148.
Go to : 
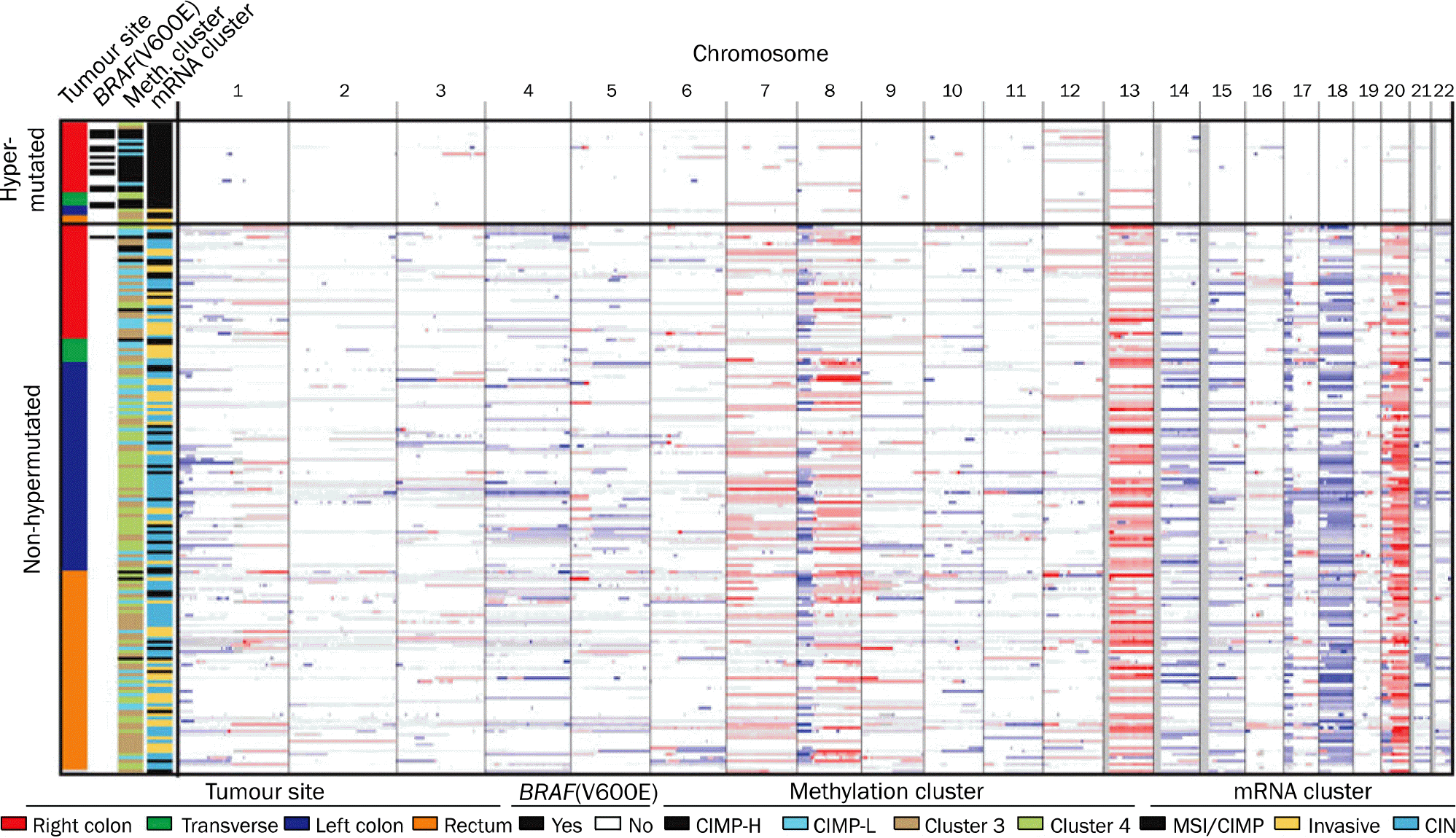 | Fig. 1.Integrative analysis of genomic changes according to the site of colon in 195 CRCs. Hypermutated cancers have near-diploid genomes and are highly enriched for hypermethylation, CIMP phenotype and BRAF (V600E) mutations. Non-hypermutated tumours originating from different sites are virtually indistinguishable from each other on the basis of their copy-number alteration patterns, DNA methylation or gene-expression patterns (citing Fig. 2 in the article of the Cancer Genome Atlas Network [Nature 2012;487:330–337]38 with the original author's permission). CIMP, CpG island methylator phenotype; H, high; L, low; MSI, microsatellite instability; CIN, chromosomal instability. |
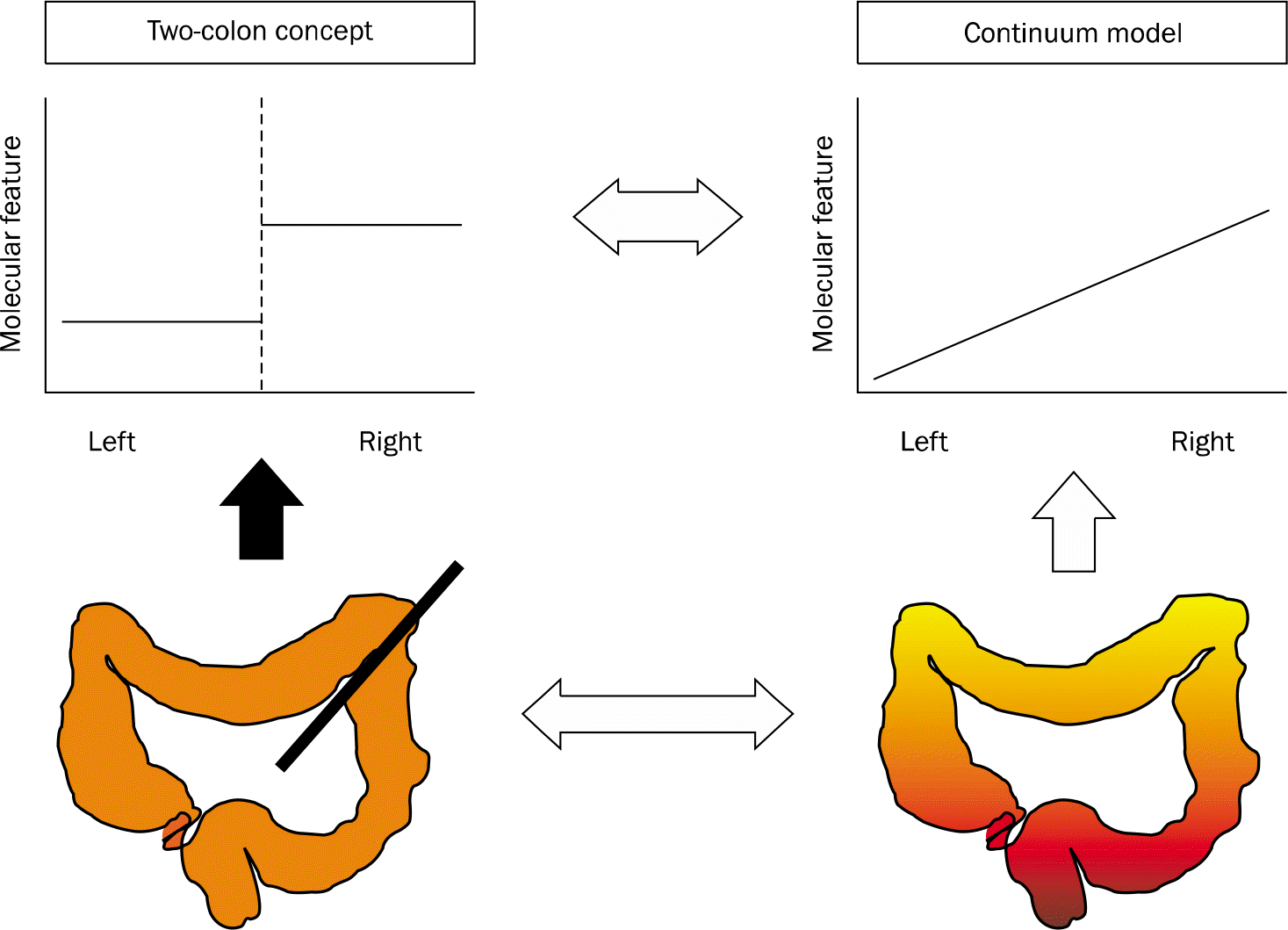 | Fig. 2.Schematic illustration of the differences between the two-colon concept and the continuum model. The molecular characteristics including a high degree of CpG island methylator phenotype (CIMP-high), a high degree of microsatellite instability (MSI-high) and BRAF mutation increase linearly from the rectum to the ascending colon according to the new continuum model. Data from numerous previous two-colon studies appear to support the two-colon concept (left, top), even when it is likely that a continuum exists for the measured marker along the proximal- distal axis of the colon (left, bottom). The design of two-colon studies does not permit adequate evaluation of the linearity of marker frequency by colon subsite (modified from Fig. 1 in the article of Yamauchi et al. [Gut 2012; 61:847–854]16 with the original author's permission). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download