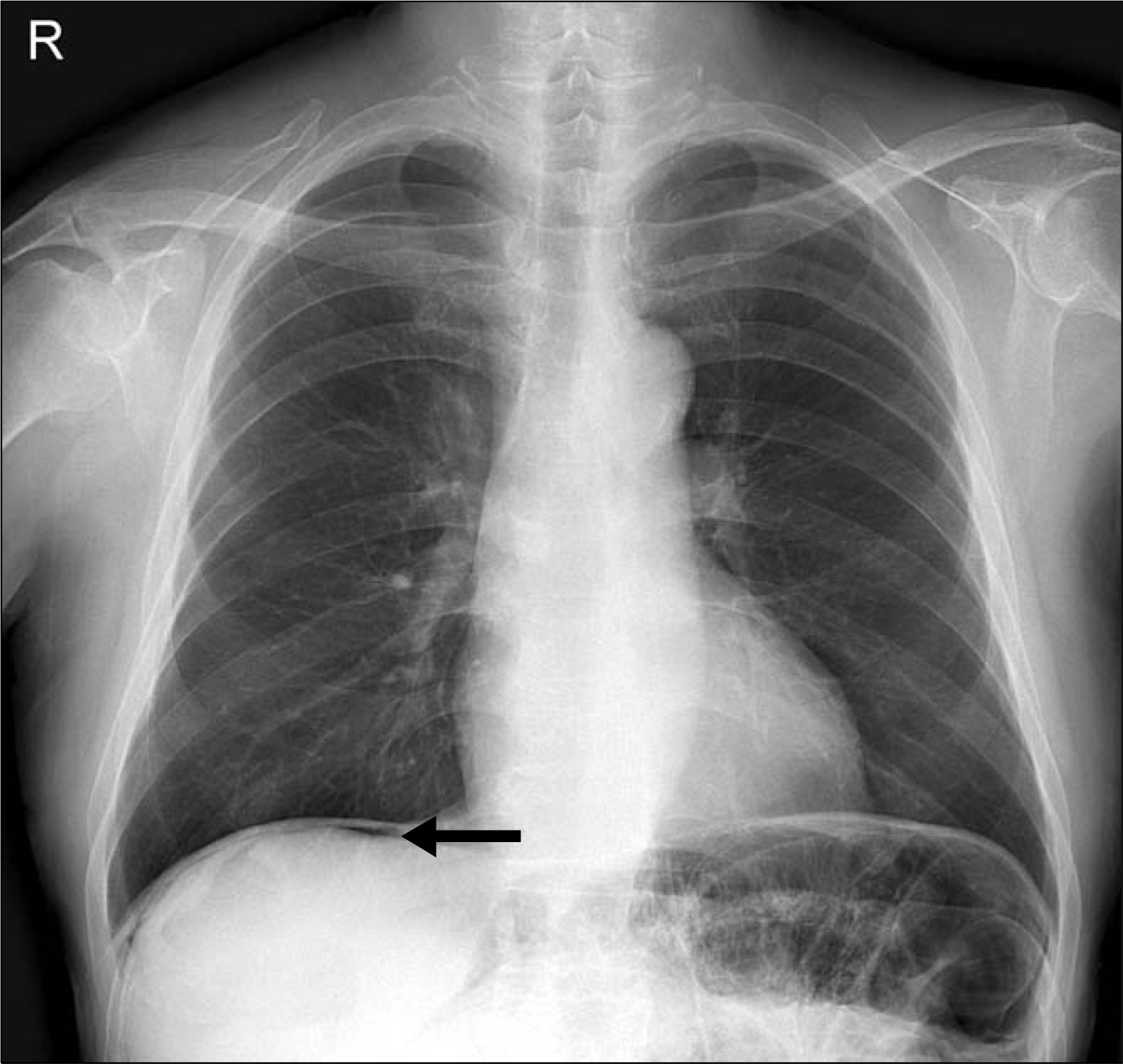
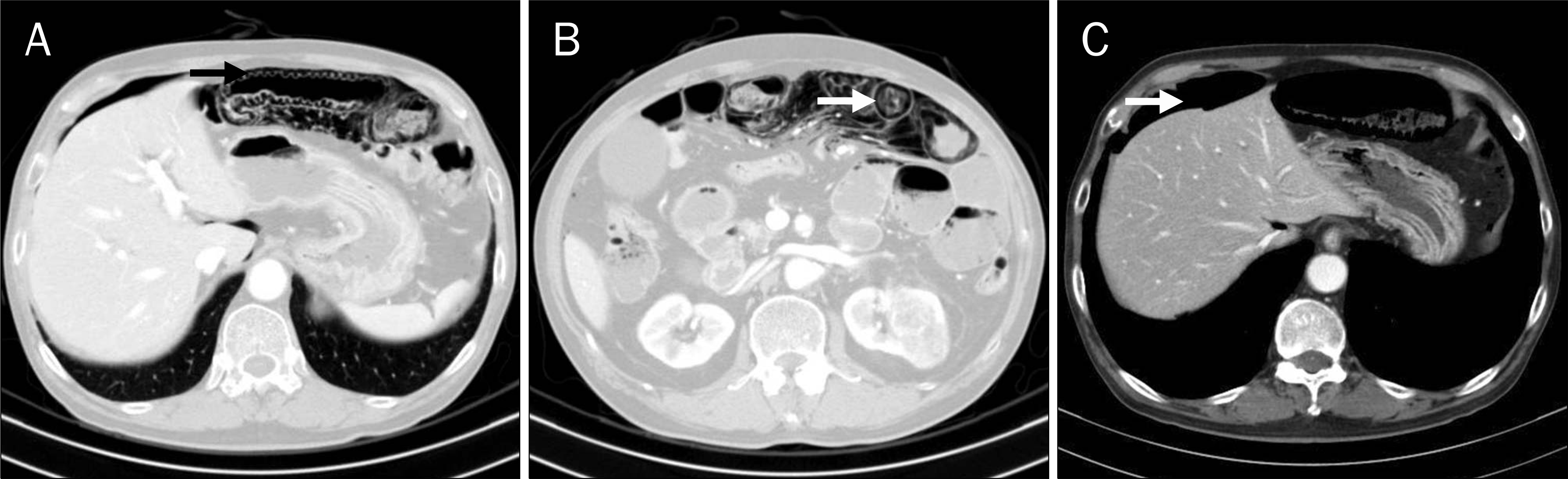
Abstract
Sunitinib as a multitarget tyrosine kinase inhibitor is one of the anti-tumor agents, approved by the United States Food and Drug Administration to use treat gastrointestinal stromal tumor and metastatic renal cell carcinoma. The agent is known to commonly induce adverse reactions such as fatigue, nausea, diarrhea, stomatitis, esophagitis, hypertension, skin toxicity, re-duciton in cardiac output of left ventricle, and hypothyroidism. However, it has been reported to rarely induce adverse reactions such as nephrotic syndrome and irreversible reduction in renal functions, and cases of intestinal perforation or pneumatosis interstinalis as such reactions have been consistently reported. In this report, a 66-year old man showing abdominal pain had renal cell carcinoma and history of sunitinib at a dosage of 50 mg/day on a 4-weeks-on, 2-weeks-off schedule. Seven days after the third cycle he was referred to the hospital because of abdominal pain. Computed tomography showed pneumoperitoneum with linear pneumatosis intestinalis in his small bowel. The patient underwent surgical exploration that confirmed the pneumatosis intestinalis at 100 cm distal to Treitz's ligament. We report a rare case of intestinal perforation with pneumatosis intestinalis after administration of sunitinib to a patient with metastatic renal cell carcinoma.
References
1. Porta C, Paglino C, Imarisio I, Bonomi L. Uncovering Pandora's vase: the growing problem of new toxicities from novel anti-cancer agents. The case of sorafenib and sunitinib. Clin Exp Med. 2007; 7:127–134.

2. Feldman DR, Martorella AJ, Robbins RJ, Motzer RJ. Re: Hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2007; 99:974–975.

3. Takahashi D, Nagahama K, Tsuura Y, Tanaka H, Tamura T. Sunitinib-induced nephrotic syndrome and irreversible renal dysfunction. Clin Exp Nephrol. 2012; 16:310–315.

4. Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006; 24:25–35.

5. Hoshino Y, Hasegawa H, Ishii Y, et al. Two cases of bowel perforation associated with sunitinib treatment for renal cell carcinoma. Int J Clin Oncol. 2012; 17:412–416.

6. Kollmannsberger C, Soulieres D, Wong R, Scalera A, Gaspo R, Bjarnason G. Sunitinib therapy for metastatic renal cell carcinoma: recommendations for management of side effects. Can Urol Assoc J. 2007; 1(2 Suppl):S41–S54.

7. Sivendran S, Liu Z, Portas LJ Jr, et al. Treatmentrelated mortality with vascular endothelial growth factor receptor tyrosine kinase inhibitor therapy in patients with advanced solid tumors: a metaanalysis. Cancer Treat Rev. 2012; 38:919–925.

8. Hur H, Park AR, Jee SB, Jung SE, Kim W, Jeon HM. Perforation of the colon by invading recurrent gastrointestinal stromal tumors during sunitinib treatment. World J Gastroenterol. 2008; 14:6096–6099.

9. Flaig TW, Kim FJ, La Rosa FG, Breaker K, Schoen J, Russ PD. Colonic pneumatosis and intestinal perforations with sunitinib treatment for renal cell carcinoma. Invest New Drugs. 2009; 27:83–87.

10. Jarkowski A 3rd, Hare R, Francescutti V, Wilkinson N, Khushalani N. Case report of pneumatosis intestinalis secondary to sunitinib treatment for refractory gastrointestinal stromal tumor. Anticancer Res. 2011; 31:3429–3432.
11. Coriat R, Ropert S, Mir O, et al. Pneumatosis intestinalis associated with treatment of cancer patients with the vascular growth factor receptor tyrosine kinase inhibitors sorafenib and sunitinib. Invest New Drugs. 2011; 29:1090–1093.

12. Raut CP, Posner M, Desai J, et al. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol. 2006; 24:2325–2331.

13. Ruka W, Rutkowski P, Nowecki Z, Dziewirski W. Emergency surgery due to complication during molecular targeted therapy in advanced gastrointestinal stromal tumors (GIST). Nowotwory. 2010; 60:1e–7e.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download