Abstract
Background/Aims
Eradication of Helicobacter pylori reduces the incidence of gastric cancer, and may inhibit gastric dysplasia progression into gastric cancer. The aim of this study was to investigate the effect of eradication of Helicobacter on the incidence of subsequent gastric dysplasia development after endoscopic resection.
Methods
Medical records of patients who underwent endoscopic resection for gastric dysplasia were retrospectively reviewed. Presence of H. pylori was assessed by the Campylobacter-like organism test and histology. The rate of subsequent dysplasia development after endoscopic resection between the eradication group and non-eradication group was compared.
Results
Total of 129 patients positive for H. pylori infection were included for analysis. Of these, 85 patients received successful eradication therapy and 44 patients did not receive eradication therapy or failed to achieve successful eradication. Sex, mean age and pathologic grade of dysplasia did not differ between the two groups. In univariate analysis, the grade of intestinal metaplasia (p=0.013) significantly differed between metachronous dysplasia group and non-metachrounous dysplasia group. In multivariate analysis, eradication of H. pylori (p=0.014) was related to reduced incidence of subsequent gastric dysplasia development after endoscopic resection.
Go to : 
References
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108.

2. Correa P, Haenszel W, Cuello C, et al. Gastric precancerous process in a high risk population: cross-sectional studies. Cancer Res. 1990; 50:4731–4736.
4. de Vries AC, Haringsma J, Kuipers EJ. The detection, surveillance and treatment of premalignant gastric lesions related to Helicobacter pylori infection. Helicobacter. 2007; 12:1–15.

5. Dinis-Ribeiro M, Areia M, de Vries AC, et al. European Society of Gastrointestinal Endoscopy; European Helicobacter Study Group; European Society of Pathology; Sociedade Portuguesa de Endoscopia Digestiva. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy. 2012; 44:74–94.
7. Fukase K, Kato M, Kikuchi S, et al. Japan Gast Study Group. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008; 372:392–397.
8. Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney system. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996; 20:1161–1181.
9. Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000; 47:251–255.

10. den Hoed CM, van Eijck BC, Capelle LG, et al. The prevalence of premalignant gastric lesions in asymptomatic patients: predicting the future incidence of gastric cancer. Eur J Cancer. 2011; 47:1211–1218.

11. Kim YJ, Park JC, Kim JH, et al. Histologic diagnosis based on forceps biopsy is not adequate for determining endoscopic treatment of gastric adenomatous lesions. Endoscopy. 2010; 42:620–626.

12. Cho SJ, Choi IJ, Kim CG, et al. Risk of high-grade dysplasia or carcinoma in gastric biopsy-proven low-grade dysplasia: an analysis using the Vienna classification. Endoscopy. 2011; 43:465–471.

13. Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006; 41:929–942.

14. Correa P. Human gastric carcinogenesis: a multistep and multi-factorial process–First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992; 52:6735–6740.
15. Saito K, Arai K, Mori M, Kobayashi R, Ohki I. Effect of Helicobacter pylori eradication on malignant transformation of gastric adenoma. Gastrointest Endosc. 2000; 52:27–32.
16. Correa P, Fontham ET, Bravo JC, et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-Helicobacter pylori therapy. J Natl Cancer Inst. 2000; 92:1881–1888.
17. You WC, Brown LM, Zhang L, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of pre-cancerous gastric lesions. J Natl Cancer Inst. 2006; 98:974–983.
18. Mera R, Fontham ET, Bravo LE, et al. Long term follow up of patients treated for Helicobacter pylori infection. Gut. 2005; 54:1536–1540.
19. Trautmann K, Stolte M, Miehlke S. Eradication of H.pylori for the prevention of gastric cancer. World J Gastroenterol. 2006; 12:5101–5107.
20. Shin HR, Jung KW, Won YJ, et al. National cancer incidence for the year 2002 in Korea. Cancer Res Treat. 2007; 39:139–149.

21. Ley C, Mohar A, Guarner J, et al. Helicobacter pylori eradication and gastric preneoplastic conditions: a randomized, double-blind, placebo-controlled trial. Cancer Epidemiol Biomarkers Prev. 2004; 13:4–10.
22. Sung JJ, Lin SR, Ching JY, et al. Atrophy and intestinal metaplasia one year after cure of H. pylori infection: a prospective, randomized study. Gastroenterology. 2000; 119:7–14.
Go to : 
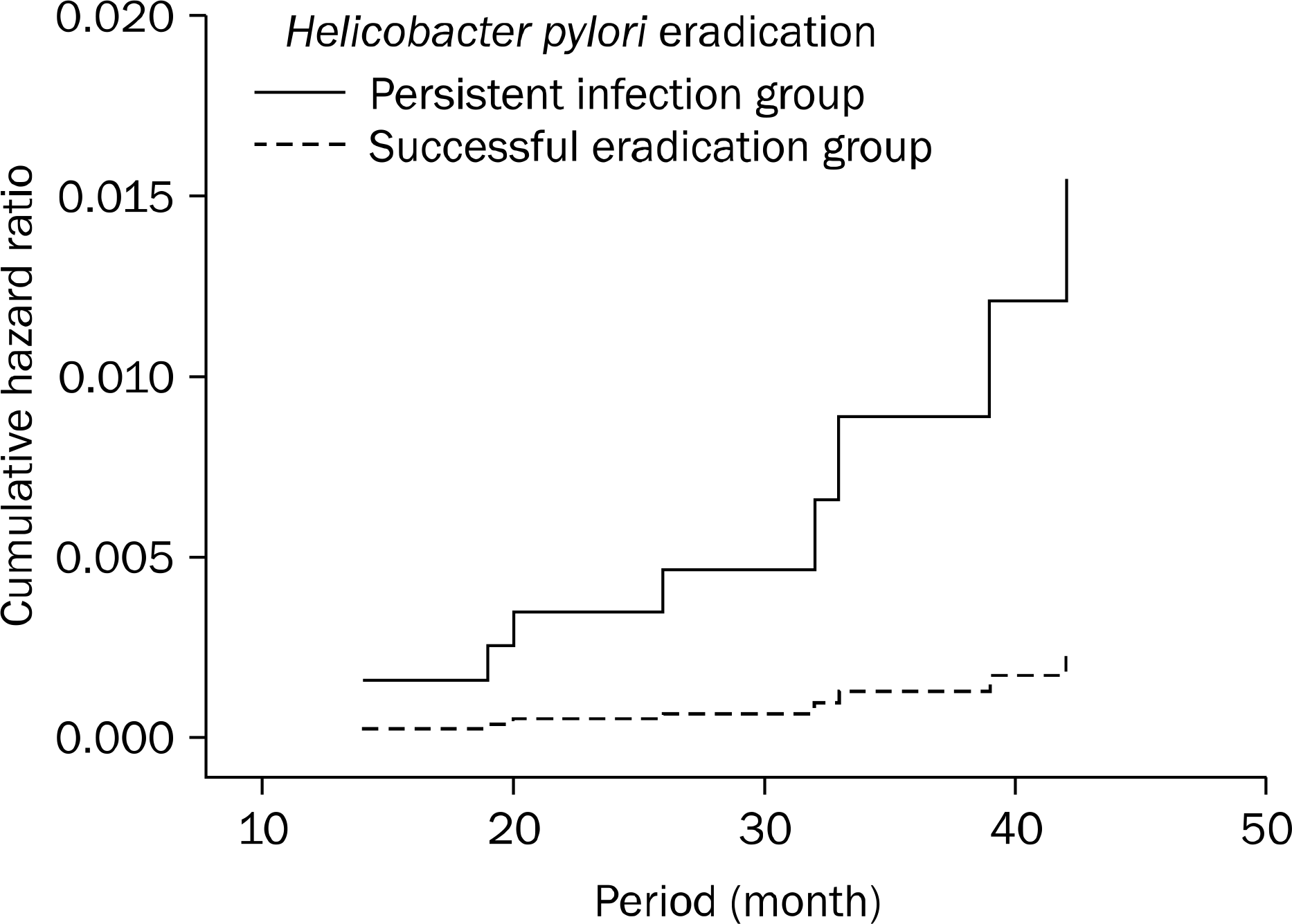 | Fig. 2.Effect of Helicobacter pylori eradication on time to development of metachronous dysplasia. |
Table 1.
Baseline Characteristics of Patients and Lesions according to Eradication Status
Table 2.
Comparison of Baseline Characteristics of Patients and Lesions between Those Who Developed Metachronous Dysplasia and Who Did Not




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download