Abstract
Background/Aims
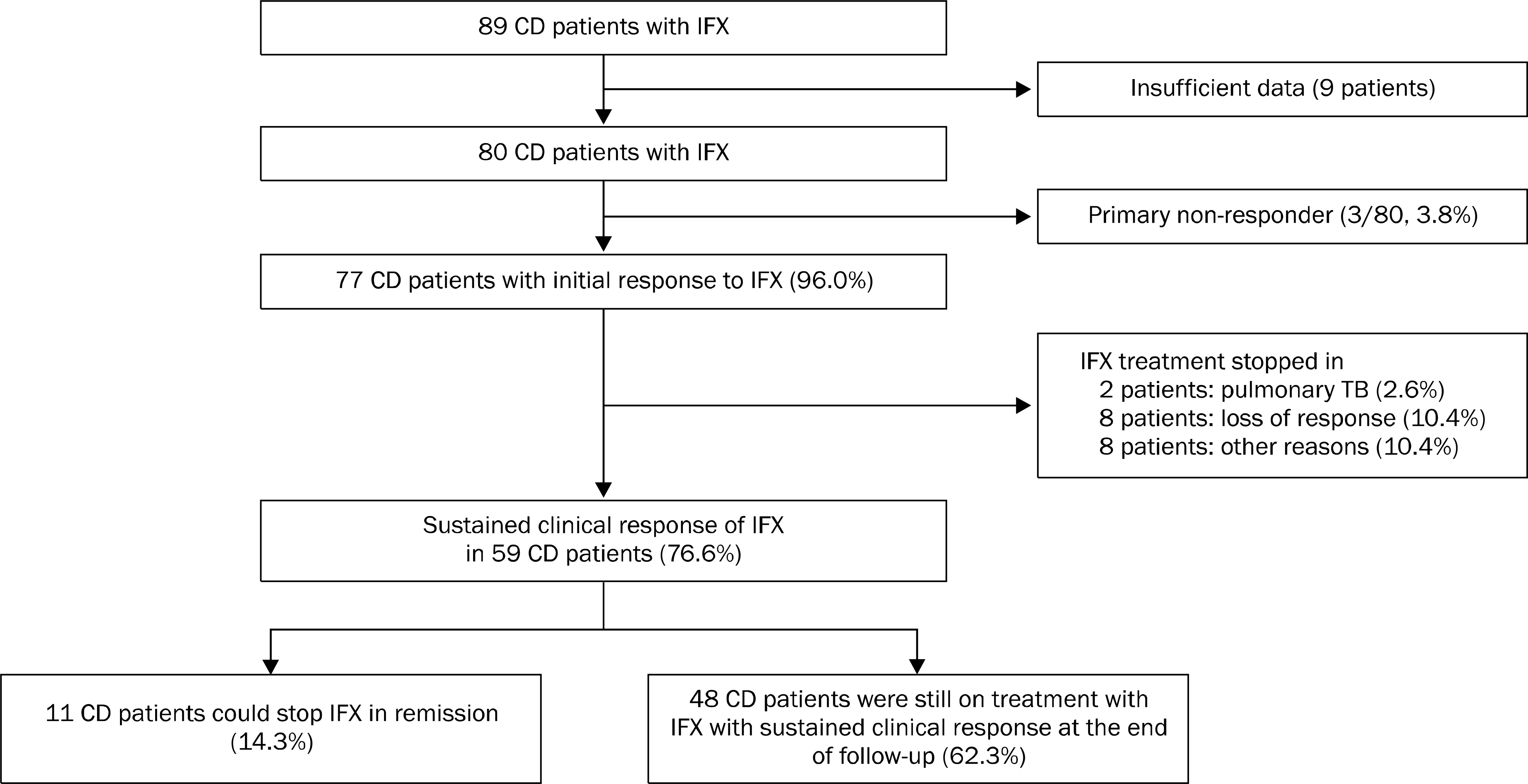
Our aim was to assess the long-term data regarding efficacy and safety of infliximab (IFX) treatment for refractory Crohn's disease (CD) patients in our tertiary teaching hospital.
Methods
We have retrospectively analyzed the medical records of 89 CD patients who underwent IFX treatment between March 2003 and February 2011 at Kyung Hee University Hospital (Seoul, Korea). The primary outcome measurements were the rates of initial clinical response (CR) at 10 weeks after the 1st IFX infusion and sustained CR at the end of the follow-up. Overall adverse events related to IFX treatment were also evaluated.
Results
The mean (SD) follow-up period of eligible 80 patients was 33.7 (21.9) months. A total of 77 patients (96%) showed initial clinical response, but 8 patients showed loss of response to IFX during the follow-up. Finally, 59 patients (59/77, 76.6%) showed sustained CR at the end of the study. Logistic regression analyses showed that an initial CR at 10 weeks was the independent predictor associated with sustained CR (OR 22.286, 95% CI 2.742–132.717, p=0.001). Overall adverse events reported in 18 patients (18/80, 23.3%), including 3 serious infection (pulmonary tuberculosis and herpes zoster).
References
1. Yang SK, Yun S, Kim JH, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986–2005: a KASID study. Inflamm Bowel Dis. 2008; 14:542–549.

2. Cosnes J, Cattan S, Blain A, et al. Longterm evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis. 2002; 8:244–250.

3. Sandborn WJ. Current directions in IBD therapy: what goals are feasible with biological modifiers? Gastroenterology. 2008; 135:1442–1447.

4. Danese S, Colombel JF, Reinisch W, Rutgeerts PJ. Review article: infliximab for Crohn's disease treatment-shifting therapeutic strategies after 10 years of clinical experience. Aliment Pharmacol Ther. 2011; 33:857–869.
5. Hanauer SB, Feagan BG, Lichtenstein GR, et al. ACCENT I Study Group. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002; 359:1541–1549.

6. Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999; 340:1398–1405.

7. Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004; 350:876–885.

8. D'Haens G, Baert F, van Assche G, et al. Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet. 2008; 371:660–667.
9. Colombel JF, Sandborn WJ, Reinisch W, et al. SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010; 362:1383–1395.

10. Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn's disease. Inflamm Bowel Dis. 2009; 15:1295–1301.

11. Schnitzler F, Fidder H, Ferrante M, et al. Longterm outcome of treatment with infliximab in 614 patients with Crohn's disease: results from a single-centre cohort. Gut. 2009; 58:492–500.

12. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006; 55:749–753.

13. Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997; 337:1029–1035.
14. Ricart E, Panaccione R, Loftus EV, Tremaine WJ, Sandborn WJ. Infliximab for Crohn's disease in clinical practice at the Mayo Clinic: the first 100 patients. Am J Gastroenterol. 2001; 96:722–729.

15. Choi KD, Song HJ, Kim JS, Jung HC, Song IS. Efficacy and safety of treatment with infliximab in Crohn's disease-the experience of single center in Korea. Korean J Gastroenterol. 2005; 46:48–55.
16. Kim SH, Yang S, Kim KJ, et al. Efficacy of infliximab in the treatment of korean patients with crohns disease. Korean J Gastroenterol. 2009; 54:108–116.

17. Rudolph SJ, Weinberg DI, McCabe RP. Longterm durability of Crohn's disease treatment with infliximab. Dig Dis Sci. 2008; 53:1033–1041.

18. Sprakes MB, Ford AC, Warren L, Greer D, Hamlin J. Efficacy, tolerability, and predictors of response to infliximab therapy for Crohn's disease: a large single centre experience. J Crohns Colitis. 2012; 6:143–153.

19. D'haens G, Van Deventer S, Van Hogezand R, et al. Endoscopic and histological healing with infliximab anti-tumor necrosis factor antibodies in Crohn's disease: A European multicenter trial. Gastroenterology. 1999; 116:1029–1034.
20. Rutgeerts P, Diamond RH, Bala M, et al. Scheduled maintenance treatment with infliximab is superior to episodic treatment for the healing of mucosal ulceration associated with Crohn's disease. Gastrointest Endosc. 2006; 63:433–442.

21. Vermeire S, Noman M, Van Assche G, Baert F, D'Haens G, Rutgeerts P. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn's disease. Gut. 2007; 56:1226–1231.

22. Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med. 2003; 348:601–608.

23. Lichtenstein GR, Diamond RH, Wagner CL, et al. Clinical trial: benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment Pharmacol Ther. 2009; 30:210–226.

24. Parsi MA, Achkar JP, Richardson S, et al. Predictors of response to infliximab in patients with Crohn's disease. Gastroenterology. 2002; 123:707–713.

25. Van Assche G, Magdelaine-Beuzelin C, D'Haens G, et al. Withdrawal of immunosuppression in Crohn's disease treated with scheduled infliximab maintenance: a randomized trial. Gastroenterology. 2008; 134:1861–1868.

26. Won HS, Park DI, Chon CU, et al. Comparison of infliximab and infliximab/azathioprine for maintenance therapy in Korean patients with luminal Crohn's disease. Intest Res. 2011; 9:189–195.

27. Mackey AC, Green L, Liang LC, Dinndorf P, Avigan M. Hepatosplenic T cell lymphoma associated with infliximab use in young patients treated for inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007; 44:265–267.

28. Beaugerie L, Brousse N, Bouvier AM, et al. CESAME Study Group. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009; 374:1617–1625.

29. Siegel CA, Marden SM, Persing SM, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn's disease: a metaanalysis. Clin Gastroenterol Hepatol. 2009; 7:874–881.

30. Ye BD, Yang SK, Shin SJ, et al. IBD Study Group of the Korean Association for the Study of the Intestinal Diseases. Guidelines for the management of Crohn's disease. Intest Res. 2012; 10:26–66.

31. Colombel JF, Loftus EV Jr, Tremaine WJ, et al. The safety profile of infliximab in patients with Crohn's disease: the Mayo clinic experience in 500 patients. Gastroenterology. 2004; 126:19–31.

32. Cheifetz A, Smedley M, Martin S, et al. The incidence and management of infusion reactions to infliximab: a large center experience. Am J Gastroenterol. 2003; 98:1315–1324.

33. Cohen RD, Tsang JF, Hanauer SB. Infliximab in Crohn's disease: first anniversary clinical experience. Am J Gastroenterol. 2000; 95:3469–3477.

34. Farrell RJ, Shah SA, Lodhavia PJ, et al. Clinical experience with infliximab therapy in 100 patients with Crohn's disease. Am J Gastroenterol. 2000; 95:3490–3497.

35. Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001; 345:1098–1104.
Fig. 2.
Clinical response at 10 weeks after the initiation of the infliximab treatment. CD, Crohn's disease.
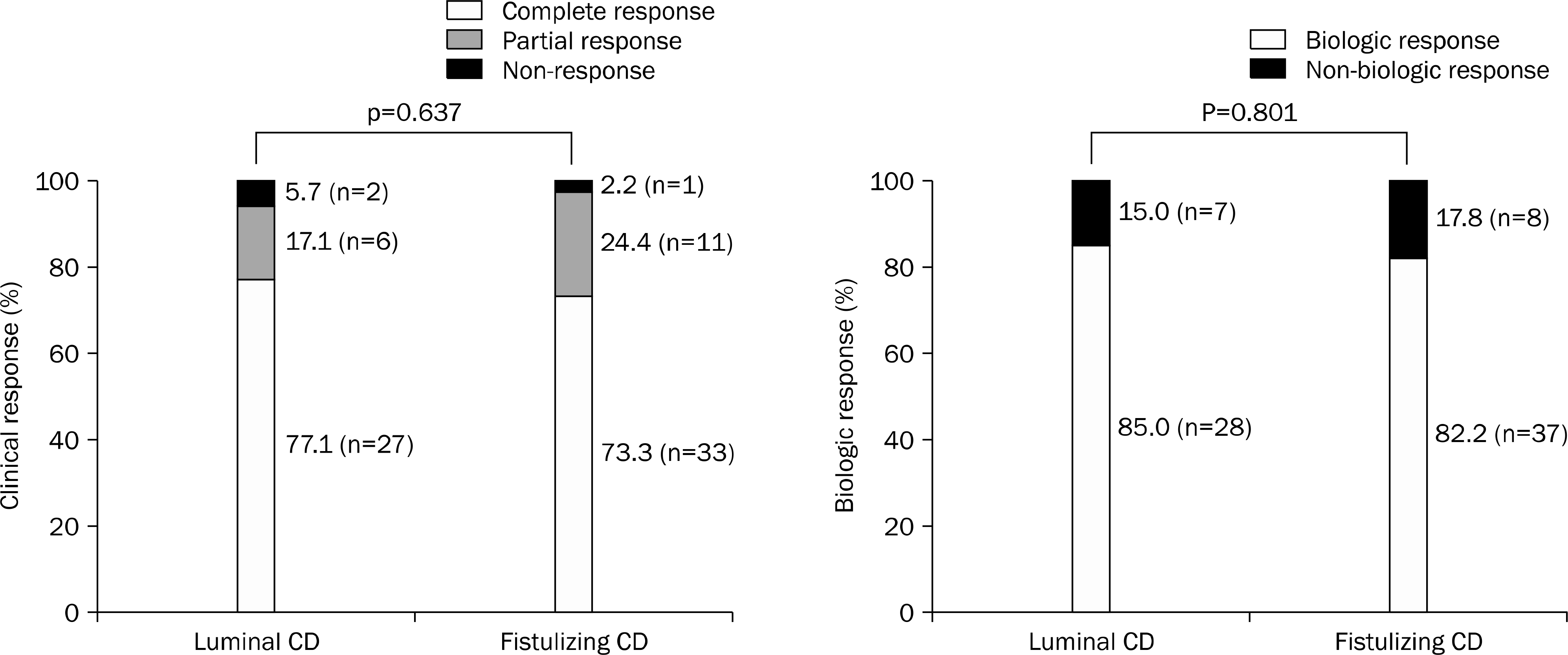
Fig. 3.
The sustained clinical and biologic response in patients with Crohn's disease (CD) at the end of follow-up.
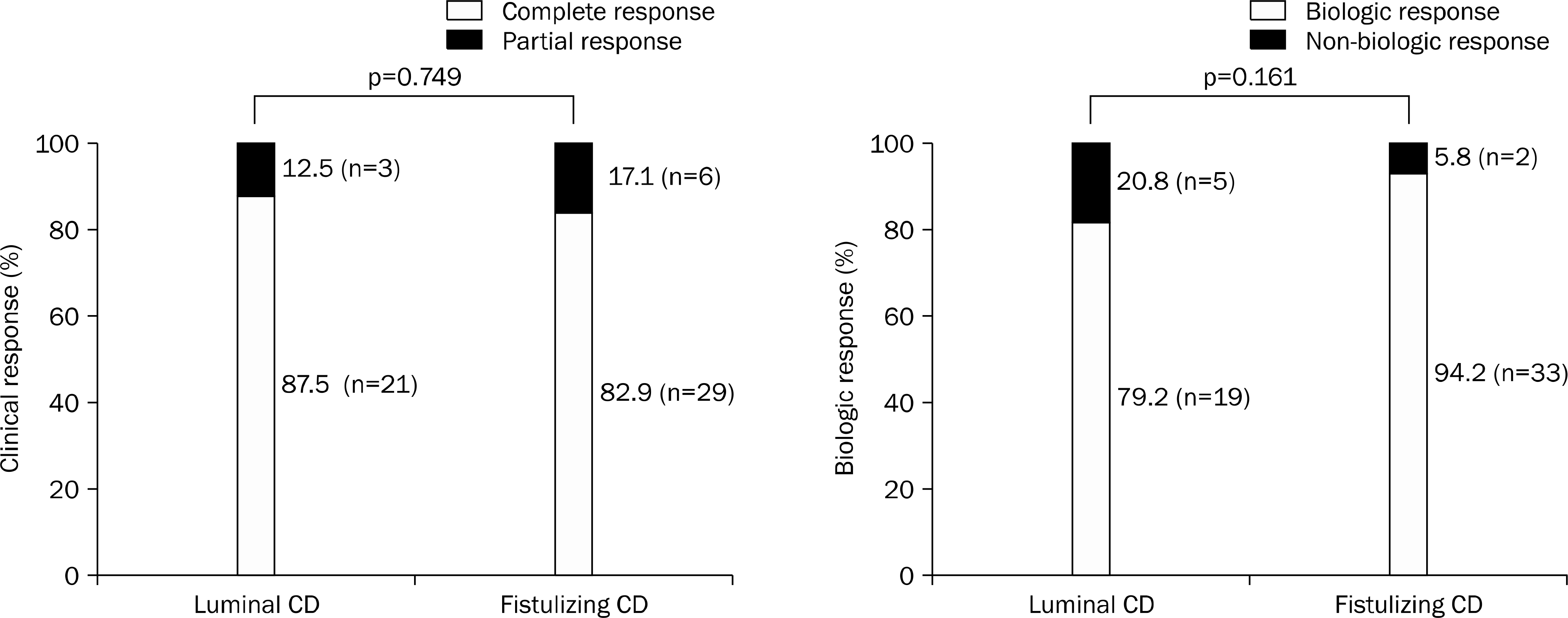
Fig. 4.
The sustained clinical response in patients with Crohn's disease at the end of follow-up stratified by of the disease prior to the infliximab (IFX) treatment (A) and clinical complete response at 10 weeks (B) via Kaplan Meier survival plots. CR, complete response; PR, partial response.
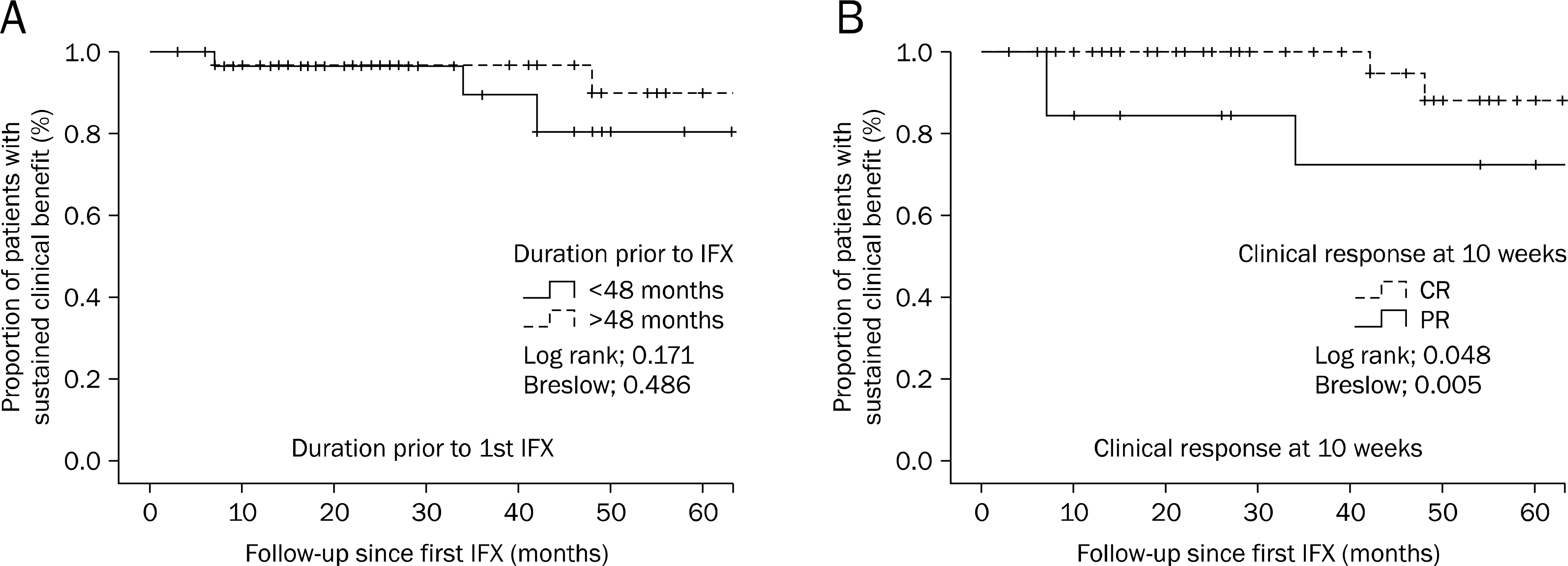
Table 1.
Patients' Baseline Characteristics
| Characteristic | Data (n=80) |
|---|---|
| Male/female | 55/25 (68.8/31.2) |
| Age (yr) | |
| At diagnosis | 27.0±8.9 |
| At the first infusion of IFX | 28.5±10.3 |
| Smoker | 14 (17.5) |
| BMI (kg/m2) | 19.8±3.4 |
| Previous abdominal surgery | 17 (21.3) |
| CDAI | 284±79 |
| CRP (mg/dL) | 3.37±3.67 |
| Montreal classification | |
| Age at diagnosis (A1/A2/A3) a | 16/58/6 (20.0/72.5/7.5) |
| Location (L1/L2/L3/L4) b | 12/18/49/1 (15.0/22.5/61.3/1.2) |
| Behavior (B1/B2/B3) c | 63/13/4 (78.8/16.2/5.0) |
| Indication for IFX treatment | |
| Luminal CD | 35 (43.8) |
| Fistulising CD | 45 (56.2) |
| Treatment schedule | |
| Episodic treatment | 14 (17.5) |
| Scheduled treatment | 66 (82.5) |
| Duration of disease prior to 1st IFX infusion (mo) | 55.6±52.6 |
| Follow up period after 1st IFX infusion (mo) | 33.7±21.9 |
| Number of infusion of IFX | 13.8±8.4 (3–37) |
| Concomitant medication at 1st IFX | |
| Aminosalicylates | 63 (78.8) |
| Steroids | 15 (18.8) |
| AZA/6-MP | 67 (83.8) |
| MTX | 1 (1.3) |
| QuantiFERON-TB Gold test | 48/7/25 (60.0/8.8/31.2) |
Table 2.
Factors Predicting Sustained Clinical Response to Infliximab Therapy by Multiple Regression Analysis
Table 3.
Adverse Events after Infliximab Treatment
| Types of adverse events | Data |
|---|---|
| Minor and transient side effects a | 13 (16.9) |
| Acute infusion reaction | 2 (2.6) |
| Serious adverse events | 3 (3.9) |
| Herpes zoster | 1 (1.3) |
| Pulmonary tuberculosis | 2 (2.6) |
| Total | 18 (23.3) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download