Abstract
Background/Aims
Endoscopic submucosal dissection (ESD) has been accepted as a standard treatment of early gastric cancer (EGC). However, the indication of ESD in undifferentiated-type EGC was controversial. The aim of this study was to evaluate the therapeutic outcomes of ESD in undifferentiated-type EGC according to expanded indication.
Methods
At Soonchunhyang University Bucheon Hospital, a total of 82 lesions in 81 patients with undifferentiated-type EGC were treated with ESD. The therapeutic outcomes of ESD were evaluated by resection method (en bloc resection; piecemeal resection), histologic curative resection, complications and recurrence rates after ESD.
Results
The rate on en bloc resection and complete resection rate were 87.8% (72/82) and 80.5% (66/82), respectively. In signet ring cell carcinoma, the complete resection rate was higher than those in poorly differentiated adenocarcinoma and poorly differentiated adenocarcinoma with signet ring cell features, but there was no statistical significance (89.3% vs.75.0%, 76.7%; p=0.347). The lateral margin positivity rate in poorly differentiated adenocarcinoma, signet ring cell carcinoma and poorly differentiated adenocarcinoma with signet ring cell features were 12.5%, 3.6% and 13.3%, respectively (p=0.395). The vertical margin positivity rate were 12.5%, 3.6% and 10.0%, respectively (p=0.485). The overall recurrence rate was 3.0% during a mean follow-up period of 37.4 months.
Go to : 
References
2. Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000; 3:219–225.

3. Tada M, Murakami A, Karita M, Yanai H, Okita K. Endoscopic resection of early gastric cancer. Endoscopy. 1993; 25:445–450.

4. Kim JH, Lee YC, Kim H, et al. Endoscopic resection for undifferentiated early gastric cancer. Gastrointest Endosc. 2009; 69:e1–e9.
5. Kang HY, Kim SG, Kim JS, Jung HC, Song IS. Clinical outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Surg Endosc. 2010; 24:509–516.

6. Yamamoto Y, Fujisaki J, Hirasawa T, et al. Therapeutic outcomes of endoscopic submucosal dissection of undifferentiated-type intramucosal gastric cancer without ulceration and preoperatively diagnosed as 20 millimetres or less in diameter. Dig Endosc. 2010; 22:112–118.

7. Kamada K, Tomatsuri N, Yoshida N. Endoscopic submucosal dissection for undifferentiated early gastric cancer as the expanded indication lesion. Digestion. 2012; 85:111–115.

8. Park J, Choi KD, Kim MY, et al. Is endoscopic resection an acceptable treatment for undifferentiated EGC? Hepatogastroenterology. 2012; 59:607–611.

9. Okada K, Fujisaki J, Yoshida T, et al. Longterm outcomes of endoscopic submucosal dissection for undifferentiated-type early gastric cancer. Endoscopy. 2012; 44:122–127.

10. Abe N, Watanabe T, Sugiyama M, et al. Endoscopic treatment or surgery for undifferentiated early gastric cancer? Am J Surg. 2004; 188:181–184.

11. Nasu J, Nishina T, Hirasaki S, et al. Predictive factors of lymph node metastasis in patients with undifferentiated early gastric cancers. J Clin Gastroenterol. 2006; 40:412–415.

12. Park YD, Chung YJ, Chung HY, et al. Factors related to lymph node metastasis and the feasibility of endoscopic mucosal resection for treating poorly differentiated adenocarcinoma of the stomach. Endoscopy. 2008; 40:7–10.

13. Ye BD, Kim SG, Lee JY, et al. Predictive factors for lymph node metastasis and endoscopic treatment strategies for undifferentiated early gastric cancer. J Gastroenterol Hepatol. 2008; 23:46–50.

14. Hamilton SR, Aaltonen LA. World Health Organization classification of tumours. Pathology and genetics. Tumours of the digestive system. Lyon: IARC Press;2000.
15. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma:3rd English edition. Gastric Cancer. 2011; 14:101–112.
16. Ohkuwa M, Hosokawa K, Boku N, Ohtu A, Tajiri H, Yoshida S. New endoscopic treatment for intramucosal gastric tumors using an insulated-tip diathermic knife. Endoscopy. 2001; 33:221–226.

17. Kurihara N, Kubota T, Otani Y, et al. Lymph node metastasis of early gastric cancer with submucosal invasion. Br J Surg. 1998; 85:835–839.

18. Yamada H, Nihei Z, Yamashita T, Shirota Y, Ichikawa W, Sugihara K. Is lymphadenectomy needed for all submucosal gastric cancers? Eur J Surg. 2001; 167:199–203.
19. Park DJ, Lee HK, Lee HJ, et al. Lymph node metastasis in early gastric cancer with submucosal invasion: feasibility of minimally invasive surgery. World J Gastroenterol. 2004; 10:3549–3552.

20. Kim JJ, Lee JH, Jung HY, et al. EMR for early gastric cancer in Korea: a multicenter retrospective study. Gastrointest Endosc. 2007; 66:693–700.
21. Okada K, Fujisaki J, Kasuga A, et al. Diagnosis of undifferentiated type early gastric cancers by magnification endoscopy with narrow-band imaging. J Gastroenterol Hepatol. 2011; 26:1262–1269.

22. Jeon SR, Cho WY, Jin SY, Cheon YK, Choi SR, Cho JY. Optical biopsies by confocal endomicroscopy prevent additive endoscopic biopsies before endoscopic submucosal dissection in gastric epithelial neoplasias: a prospective, comparative study. Gastrointest Endosc. 2011; 74:772–780.

23. Kumarasinghe MP, Lim TK, Ooi CJ, Luman W, Tan SY, Koh M. Tubule neck dysplasia: precursor lesion of signet ring cell carcinoma and the immunohistochemical profile. Pathology. 2006; 38:468–471.

24. Mouri R, Yoshida S, Tanaka S, Oka S, Yoshihara M, Chayama K. Usefulness of endoscopic ultrasonography in determining the depth of invasion and indication for endoscopic treatment of early gastric cancer. J Clin Gastroenterol. 2009; 43:318–322.

Go to : 
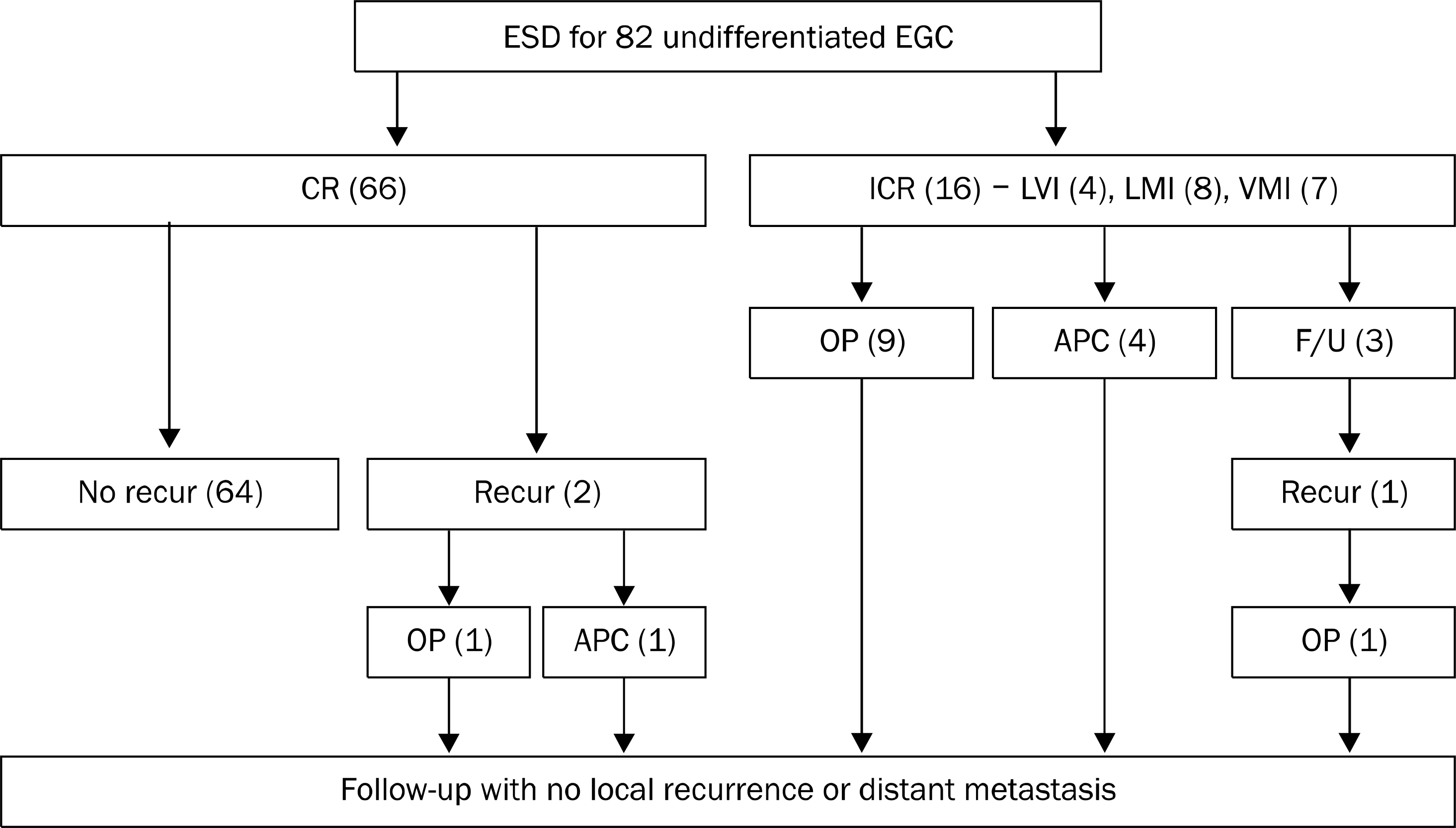 | Fig. 1.Clinical courses after ESD in undifferentiated EGC. ESD, endoscopic submucosal dissection; EGC, early gastric cancer; CR, complete resection; ICR, incomplete resection; LVI, lymphovascular invasion; LMI, lateral margin invasion; VMI, vertical margin invasion; OP, operation; APC, argon plasma coagulation; F/U, fo-llow-up; Recur, recurrence. |
Table 1.
Clinical Characteristics of Undifferentiated EGC before ESD
Table 2.
Clinical Results after ESD in Undifferentiated EGC
Values are presented n (%). ESD, endoscopic submucosal dissection; EGC, early gastric cancer; PD, poorly differentiated adenocarcinoma; SRC, signet ring cel carcinoma; PD+SRC, poorly differentiated adenocarcinoma with signet ring cell features; ICR, incomplete resection; CR, complete resection APC, argon plasma coagulation.
Table 3.
Relationship between Clinicopathologic Factors and Histologically Complete Resection




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download