Abstract
Background/Aims
Probiotics are live non-pathogenic organisms that belong to the resident microflora, and confer health benefits by multiple mechanisms. Lactobacillus rhamnosus GG (LGG) is one of the probiotic bacteria that ameliorates intestinal injury and inflammation caused by various stimuli. We aimed to evaluate the anti-inflammatory effect and mechanism of LGG in lipopolysaccharide (LPS)-stimulated HT-29 cells.
Methods
HT-29 cells were stimulated with interleukin (IL)-1β (2 ng/mL), tumor necrosis factor (TNF)-α (20 ng/mL), and LPS (20 µg/mL) in the presence or absence of LGG (107-109 colony forming units/mL). Production of the pro-inflammatory chemokine IL-8 was measured by ELISA and semi-quantitative PCR. Transcriptional activity of NF-κB-responsive gene was evaluated by luciferase assay with reporter gene. Toll-like receptor 4 (TLR4) mRNA expression was assessed by semi-quantitative PCR. The IκBα degradation was evaluated by western blot and intranuclear translocation of NF-κB was determined by western blot and immunofluorescence.
Results
LGG did not affect the viability of HT-29 cells. Pretreatment of HT-29 cells with LGG significantly blocked TNF-α, and LPS induced IL-8 activation at both mRNA and protein level (p<0.05). Pretreatment of HT-29 cells with LGG attenuated LPS-induced NF-κB nuclear translocation and also blocked LPS-induced IκBα degradation. LGG also down-regulated TLR4 mRNA activated by LPS.
Figures and Tables
Fig. 1
Lactobacillus rhamnosus GG (LGG) did not affect the viability of HT-29 cell. HT-29 cells were seeded at the density of 1×104 cells/well in 6-well plates and maintained in the medium with 10% FBS for 24 hours. (A) LGG were added to the HT-29 cell culture wells at the appropriated dilution to reach a final concentration of 105, 106, 107, 108, 109, and 1010 colony forming units (CFU) per mL of the incubation medium without antibiotics. After 4 hours incubation, cell viability was determined by MTT assay. (B) The 109 CFU/mL concentration of LGG were added to HT-29 cell culture well and incubated with various time intervals (6, 12, 18, and 24 hours). Data are the mean of triplicate assays and represent the relative viability compared to untreated controls.
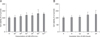
Fig. 2
Attenuation of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, or lipopolysaccharide (LPS)-mediated suppression of IL-8 expression by Lactobacillus rhamnosus GG (LGG) in HT-29 cells. (A) HT-29 cells were pre-incubated with LGG (1×109 colony forming units [CFU]/well) for 1 hour before treatment with 20 ng/mL of TNF-α, 2 ng/mL of IL-1β, and 20 µg/mL of LPS. Supernatant were harvested for IL-8 ELISA. (B) HT-29 cells were pre-incubated with different concentration of LGG (1×107, 1×108, and 1×109 CFU/mL) for 1 hour before treatment with 20 µg/mL of LPS. Supernatant were harvested for IL-8 ELISA. (C) Cells were harvested for RT-PCR analysis of IL-8 mRNA expression. GAPDH expression was used as control. The increases in the percentages of IL-8 mRNA expression were quantified by densitometric analysis.
*p<0.05, **p<0.01 in Mann-Whitney U test.

Fig. 3
Attenuation of lipopolysaccharide (LPS)-mediated induction of TLR-4 expression by Lactobacillus rhamnosus GG (LGG) in HT-29 cells. HT-29 cells were pre-incubated with different concentration of LGG (1×107, 1×108, and 1×109 CFU/well) for 1 hour before treatment with 20 ng/mL of tumor necrosis factor (TNF)-α, 2 ng/mL of interleukin (IL)-1β, and 20 µg/mL of LPS. Cells were harvested for RT-PCR analysis of toll-like receptor 4 (TLR4) mRNA expression. GAPDH expression was used as control. The increases in the percentages of TLR4 mRNA expression were quantified by densitometric analysis.
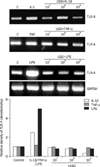
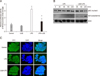
Fig. 4
Effect of Lactobacillus rhamnosus GG (LGG) on NF-κB transcriptional activation induced by lipopolysaccharide (LPS). (A) SW480 cells were cotransfected with an HIV-1 long-terminal repeat luciferase construct containing NF-κB binding sites and pCMV β-gal plasmid. pCMV β-gal served as a marker of transfection efficiency. Cotransfected cells were stimulated with LPS (20 µg/mL) with or without pretreatment of LGG (1×109 colony forming units [CFU]/mL), and NF-κB-dependent luciferase activity was measured 4 hours after stimulation. Data represent the mean with SEM and are expressed as fold increase over the media control cells. Results are expressed as means of triplicate determinations and are representative of three independent experiments. *p<0.05 compared with LPS-stimulated cells without LGG. (B) HT-29 cells were lysed at different times (15, 30, 60 and 120 minutes) after LPS (20 µg/mL) stimulation with or without pretreatment of LGG (1×109 CFU/mL). Nuclear and cytoplasmic fractions were separately prepared for Western blot. Samples were resolved by SDS-PAGE and analyzed by Western blotting with anti-NFκB/p65, anti-IκBα antibody and an anti-β-actin antibody for control. (C) HT-29 cell were plated in 4-chamber slides, grown to 70% confluence, and pre-treated with or without LGG (1×109 CFU/mL). LPS (20 µg/mL) was added to the medium and incubation continued for 4 hours. Fluorescein isothiocyanate (FITC) conjugated secondary antibody and 4',6-diamidino-2-phenylindole (DAPI) was used for immunofluorescence. DAPI staining served to visualize the nucleus (×500). These results are representative of three independent experiments.
Notes
References
1. Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989. 66:365–378.
2. Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004. 126:1620–1633.
3. Venturi A, Gionchetti P, Rizzello F, et al. Impact on the composition of the faecal flora by a new probiotic preparation: preliminary data on maintenance treatment of patients with ulcerative colitis. Aliment Pharmacol Ther. 1999. 13:1103–1108.
4. Mack DR, Michail S, Wei S, McDougall L, Hollingsworth MA. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am J Physiol. 1999. 276:G941–G950.
5. Flynn S, van Sinderen D, Thornton GM, Holo H, Nes IF, Collins JK. Characterization of the genetic locus responsible for the production of ABP-118, a novel bacteriocin produced by the probiotic bacterium Lactobacillus salivarius subsp. salivarius UCC118. Microbiology. 2002. 148:973–984.
6. Madsen K, Cornish A, Soper P, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001. 121:580–591.
7. Haller D, Bode C, Hammes WP, Pfeifer AM, Schiffrin EJ, Blum S. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut. 2000. 47:79–87.
8. Helgeland L, Vaage JT, Rolstad B, Midtvedt T, Brandtzaeg P. Microbial colonization influences composition and T-cell receptor V beta repertoire of intraepithelial lymphocytes in rat intestine. Immunology. 1996. 89:494–501.
9. Zocco MA, dal Verme LZ, Cremonini F, et al. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 2006. 23:1567–1574.
10. McFarland LV. Evidence-based review of probiotics for antibiotic-associated diarrhea and Clostridium difficile infections. Anaerobe. 2009. 15:274–280.
11. Kumar A, Wu H, Collier-Hyams LS, et al. Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species. EMBO J. 2007. 26:4457–4466.
12. Do VT, Baird BG, Kockler DR. Probiotics for maintaining remission of ulcerative colitis in adults. Ann Pharmacother. 2010. 44:565–571.
13. Testro AG, Visvanathan K. Toll-like receptors and their role in gastrointestinal disease. J Gastroenterol Hepatol. 2009. 24:943–954.
14. Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007. 449:819–826.
15. Lee SK, Kim TI, Kim YK, et al. Cellular differentiation-induced attenuation of LPS response in HT-29 cells is related to the down-regulation of TLR4 expression. Biochem Biophys Res Commun. 2005. 337:457–463.
16. Gorbach SL. Lactic acid bacteria and human health. Ann Med. 1990. 22:37–41.
17. Cain AM, Karpa KD. Clinical utility of probiotics in inflammatory bowel disease. Altern Ther Health Med. 2011. 17:72–79.
18. Jones RM, Wu H, Wentworth C, Luo L, Collier-Hyams L, Neish AS. Salmonella AvrA coordinates suppression of host immune and apoptotic defenses via JNK pathway blockade. Cell Host Microbe. 2008. 3:233–244.
19. Giahi L, Aumueller E, Elmadfa I, Haslberger AG. Regulation of TLR4, p38 MAPkinase, IκB and miRNAs by inactivated strains of lactobacilli in human dendritic cells. Benef Microbes. 2012. 3:91–98.
20. Nandakumar NS, Pugazhendhi S, Madhu Mohan K, Jayakanthan K, Ramakrishna BS. Effect of Vibrio cholerae on chemokine gene expression in HT29 cells and its modulation by Lactobacillus GG. Scand J Immunol. 2009. 69:181–187.
21. Donato KA, Gareau MG, Wang YJ, Sherman PM. Lactobacillus rhamnosus GG attenuates interferon-{gamma} and tumour necrosis factor-alpha-induced barrier dysfunction and pro-inflammatory signalling. Microbiology. 2010. 156:3288–3297.
22. Vizoso Pinto MG, Rodriguez Gómez M, Seifert S, Watzl B, Holzapfel WH, Franz CM. Lactobacilli stimulate the innate immune response and modulate the TLR expression of HT29 intestinal epithelial cells in vitro. Int J Food Microbiol. 2009. 133:86–93.
23. Hamilton MJ, Snapper SB, Blumberg RS. Update on biologic pathways in inflammatory bowel disease and their therapeutic relevance. J Gastroenterol. 2012. 47:1–8.
24. Oostenbrug LE, Drenth JP, de Jong DJ, et al. Association between toll-like receptor 4 and inflammatory bowel disease. Inflamm Bowel Dis. 2005. 11:567–575.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download