Abstract
Colloid carcinoma of the liver is very rare, and its clinicopathologic features have not been well characterized yet. We describe herein a case of colloid carcinoma of the liver. Imaging revealed a lobulated mass, measuring 12 cm in diameter at the right lobe of the liver with direct invasion of adjacent peripheral intrahepatic bile ducts. Right hemihepatectomy of the liver was performed according to the possibility of the tumor's malignant behavior. Histopathological examination of the specimen revealed large extracelluar stromal mucin pools containing floating cuboidal to columnar neoplastic cells without ovarian-like stroma. This case seemed to be colloid carcinoma arising in association with intraductal papillary neoplasm of the liver.
By convention, colloid carcinoma (CC) is defined as the presence, more than 50% of neoplasm, of large extracellular stromal mucin pools containing scanty floating carcinoma cells.1 It is a well-defined entity in the breast or large bowel.2,3 However, CC of the liver is very rare and its clinicopathologic features have not been well characterized yet. To our best knowledge, CC of the liver is not reported in Korea. We herein report a case of CC arising in association with intraductal papillary neoplasm (IPN) of the liver successfully treated by surgical resection.
A 75-year-old woman was referred to our hospital for the evaluation of large-sized right lobe hepatic mass detected by the health examination at local clinic. She had three hepatic cysts found in the liver ultrasonography five years ago considered benign. She had hypertension diagnosed one year ago without any other past medical histories. She did not smoke nor drink. Her physical examination was unremarkable and general condition was well. The results of laboratory studies showed elevation of serum gamma glutamyl transferase level to 90 IU/L (reference value 0-32 IU/L). The remainder of her serum chemistries, complete blood count, and coagulation profile were normal. In viral hepatitis test panel, absence of HBsAg, HBsAb, and anti-HCV were presented. Serum level of tumor markers such as carcinoembryonic antigen, CA 19-9, and AFP showed 2.44 mg/L, 22.39 U/mL, and 3.25 mg/L which were within normal limits.
Abdominal CT demonstrated a lobulated mass measuring 12.0 cm in diameter at the right lobe of the liver with direct invasion of adjacent peripheral intrahepatic bile ducts and variable sized simple cysts, largest one at the right posterosuperior segment measuring about 7.4 cm (Fig. 1). Hemodynamic CT showed early rim enhancement with slow central enhancement on delayed image, similar to cholangiocarcinoma. Consecutive liver biopsy revealed atypical glandular hyperplasia and mucinous cystic neoplasm, which was uncertain due to scant amount of specimen for diagnosis but suspicious of premalignant lesion. At 2 months of follow-up, MRI (Fig. 2) also revealed a low attenuating mass as high in the T2-weighted image with mild enhancement of numerous septa, and irregular large cystic portion. The mass communicated to intrahepatic duct and increased in size from 12.0 cm to 14.6 cm compared to previous CT.
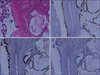
Right hemihepatectomy of the liver was performed because the lesion seemed to be a potential malignancy. Gross examination of the resected specimens revealed well demarcated solid mucoid mass without capsule, measuring 13.0×12.0×10.0 cm in size with multiple small papillary nodules in the lumen of the bile duct (Fig. 3). Microscopically, the dilated bile duct was lined by papillary-villous growing intraepithelial neoplasia (intermediate to high grade). Also large extracelluar stromal mucin pools containing floating cuboidal to columnar neoplastic cells, in more than 50% of the neoplasm were noted (Fig. 4A). Ovarian-like stroma was not observed in the wall. On immunohistochemisty using CK20 (dilution 1:1,000; Dako, Glostrup, Denmark), mucin expression such as mucin core protein (MUC) 1, MUC2, MUC5AC, and MUC6 (dilution 1:200; NovoCastra, Newcastle, UK) MUC5AC and MUC6 was positive and others were negative (Fig. 4B-D). The patient remained well with no evidence of post operation complication or recurrence for 6 months since her operation.
CC is characterized by dissecting nodules of more than 50% extracelluar mucin that contains scanty malignant cells, and is well defined entity in the breast and large bowel. Although colloid (mucinous noncystic) carcinoma of the pancreas is acceptable as a distinct disease entity, the concept of a CC of the liver has not been well-established because its reported case is very rare.
Sharing embryonic origin of the two duct systems,4 there are a number of parallels between intraductal papillary neoplasm of the bile ducts and intraductal papillary mucinous neoplasm (IPMN) of the pancreas.5 The recognition of biliary papillary neoplasm with respect to pancreatic equivalent may lead to a better understanding. Both lesions are papillary and intraductal, and both show similar range of possible differentiation of the neoplastic cells. The new World Health Organization (WHO) classification of tumors (2010) lists "IPN with an associated invasive carcinoma of the intrahepatic bile ducts" and "IPMN with an associated invasive carcinoma of the pancreas", respectively.
CC of the pancreas arises in association with an intestinal-type IPMN, while CC of the liver usually arises in association with gastric type IPN.6 IPN of the bile duct is proposed for precursor of intrahepatic cholangiocarcinoma (ICC).7 From new WHO classification of tumors (2010), CC of the pancreas is classified as separate variants of ductal adenocarcinoma, while CC of the liver and rare variants of ICC have not been listed as a separate disease entity. From current WHO classification, our case can be included to "IPN with an associated invasive carcinoma".
In our case, imaging study required differential diagnosis of biliary mucinous cystic neoplasm such as biliary cystadenoma and cystadenocarcinoma. The mucinous cystic neoplasm usually does not communicate with ductal system and is composed of columnar, mucin producing epithelium associated with ovarian-type subepithelial stroma.
IPN of the bile duct has four distinct histological subtypes including pancreaticobiliary, intestinal, oncocytic, and gastric type. Recent retrospective study represent that tumors were more often malignant in intestinal and pacreaticobiliary type than in gastric type IPN of the bile duct.8 With immuohistochemical stains, gastroenteric metaplasia shows positive expression of CK20, MUC2, MUC5AC, and/or MUC6, while intestinal type showed strong expression of CDX2 and MUC2.9 Our case showed positive MUC5AC and MUC6 expression, while MUC1, negative MUC2 and CK20 expression.
In our case, luminal communication with the bile ducts and absence of ovarian-type stroma and positive expression of MUC5AC and MUC6 support the diagnosis of IPN. From pathologic examination, CC arises in association to bile duct IPN associated with gastric type metaplasia of the liver.
Prognosis of CC of the liver has not been determined. In case of IPN of the bile duct, tumors are associated to better outcome compared with nonpapillary bile duct carcinoma and may represent an alternative carcinogenesis pathway in the biliary tract.5 In case of CC of pancreas, it is considered as a distinct pancreatic neoplasm with good prognosis, 5-year survival rate reaching 60%.1 Our patient remained well with no evidence of post operation complication or recurrence for 6 months since her operation.
We demonstrate rare case of intrahepatic CC, showing MUC1-negative and MUC6-positive expression. If more cases of CC of the liver are reported, consensus could achieved regarding the preoperative evaluation and treatment plans.
Figures and Tables
Fig. 1
CT revealed a lobulated mass measuring 12.0 cm in diameter at the right lobe of the liver (A) and variable sized simple cysts, largest one at the right posterosuperior segment measuring about 7.4 cm (B).
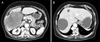
Fig. 2
MRI revealed a low attenuating mass as high in the T2-weighted image with mild enhancement of numerous septa and irregular large cystic portion.
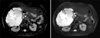
Fig. 3
The gross appearance of the resected specimen showed well demarcated solid mucoid mass without capsule, measuring 13.0×12.0×10.0 cm in size with multiple small papillary nodules in the lumen of the bile duct.

Fig. 4
Microscopic findings (×40). (A) Intraductal papillary neoplasm (IPN; left side) and colloid carcinoma (CC; right side) (H&E, ×40). (B) IPN and CC showed mucin core protein (MUC) 6 positive (MUC6 immunohistochemical stain, ×40). (C) IPN and CC showed MUC5AC positive (MUC5AC immunohistochemical stain, ×40). (D) IPN and CC showed MUC2 negative (MUC2 immunohistochemical stain, ×40).
References
1. Adsay NV, Pierson C, Sarkar F, et al. Colloid (mucinous noncystic) carcinoma of the pancreas. Am J Surg Pathol. 2001. 25:26–42.
2. Silverberg SG, Kay S, Chitale AR, Levitt SH. Colloid carcinoma of the breast. Am J Clin Pathol. 1971. 55:355–363.
3. Minsky BD, Mies C, Rich TA, Recht A, Chaffey JT. Colloid carcinoma of the colon and rectum. Cancer. 1987. 60:3103–3112.
4. Sadler TW. Langman's medical embryology. 2006. 10th ed. Philadelphia: Williams & Wilkins.
5. Rocha FG, Lee H, Katabi N, et al. Intraductal papillary neoplasm of the bile duct: A biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology. 2012. 56:1352–1360.
6. Shibahara H, Tamada S, Goto M, et al. Pathologic features of mucin-producing bile duct tumors: two histopathologic categories as counterparts of pancreatic intraductal papillary-mucinous neoplasms. Am J Surg Pathol. 2004. 28:327–338.
7. Nakanishi Y, Zen Y, Kondo S, Itoh T, Itatsu K, Nakanuma Y. Expression of cell cycle-related molecules in biliary premalignant lesions: biliary intraepithelial neoplasia and biliary intraductal papillary neoplasm. Hum Pathol. 2008. 39:1153–1161.
8. Kim KM, Lee JK, Shin JU, et al. Clinicopathologic features of intraductal papillary neoplasm of the bile duct according to histologic subtype. Am J Gastroenterol. 2012. 107:118–125.
9. Nakanuma Y, Sasaki M, Ishikawa A, Tsui W, Chen TC, Huang SF. Biliary papillary neoplasm of the liver. Histol Histopathol. 2002. 17:851–861.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download