Abstract
The term inflammatory pseudotumor (IPT) has been used to describe inflammatory and fibrosing tumoral processes of an undetermined cause that may involve a variety of organ system. IgG4-related disease is a newly recognized fibroinflammatory condition characterized by IgG4-producing plasma cell expansion in affected organs and, often but not always, elevated serum IgG4 concentrations. IgG4-related IPTs, a subtype of IPT, are characterized by dense infiltration of IgG4-positive plasma cells and stromal fibrosis. The association between inflammatory pseudotumor and IgG4 was first reported with a regard to sclerosing pancreatitis. Despite there are many reports on intraperitoneal IPTs including both cellular and lymphoplasmacytic type, only a few cases have been confirmed to be IgG4-related. We experienced a case of intraperitoneal IgG4-related inflammatory pseudotumor in an 83-year-old woman presenting with epigastric pain and malaise. Surgical specimens revealed an IgG4-related inflammatory pseudotumor.
The term inflammatory pseudotumor (IPT) has been used to describe inflammatory and fibrosing tumoral processes of an undetermined cause that may involve a variety of organ system. IgG4-related IPTs, a subtype of IPT, are characterized by dense infiltration of IgG4-positive plasma cells and stromal fibrosis. Despite there are many reports on intraperitoneal IPTs including both cellular and lymphoplasmacytic type, only a few cases have been confirmed to be IgG4-related.1-4 Previously reported cases of IgG4-related IPT were with a form of sclerosing mesenteritis2,3 or IPTs associated with other parenchymal IgG4-related sclerosing disorders.1,4 We like to present a case of a single well circumscribed intraperitoneal IgG4-related IPT in Korea for the first time.
An 83-year-old woman was referred to our hospital for further evaluation of abdominal mass. She had complained of epigastric pain and malaise for 2 weeks. There was no history of previous illness. On physical examination, a mass was palpable on the left upper quadrant of the abdomen. The peripheral blood test results were as follows: a white blood cells level of 6,510/µL (neutrophil 65.9%, lymphocyte 26.0%, monocyte 5.4%); a hemoglobin level of 10.0 g/dL; a platelet level of 302×103/µL; a CRP level of 76.7 mg/L; and an erythrocyte sedimentation rate level of 64 mm/hour. The serum amylase level and biochemical tests were normal. Chest x-ray and upper endoscopic examination was unremarkable. An 11×8 cm sized well defined heterogenous mass was found on CT scan (Fig. 1). A subsequent13 fludeoxyglucose (FDG)-PET scan revealed a mild but homogenous FDG uptake at primary mass with no evidence of distant metastasis.
We decided to perform open laparotomy to rule out malignancy. A large encapsulated mass originated from the left gastrocolic ligament, was displacing between the stomach, spleen, pancreas, and distal transverse colon. Tumorectomy was done without complication. Recovery was uneventful and the patient was discharged on day 8 postoperatively. Grossly, the tumor was 10.5×6.5×3.5 cm sized round solid mass with smooth border and the cut surface was pale yellowish white and rubbery with streaks (Fig. 2). Microscopically, the tumor was composed of cytologically bland spindle shaped cells loosely arranged in a fibrotic stroma and a prominent lymphoplasmacytic infiltrate, occasionally with formation of germinal centers. Immunohistochemical stains revealed many IgG4-positive plasma cells (average 20 per high power field; Fig. 3). The spindle cells were strongly positive for vimentin and smooth muscle actin and negative for anaplastic lymphoma kinase (ALK), CD34, and CD117. These pathological features were compatible with IgG4-related IPT. After surgery, CRP level was normalized. Preoperative serum IgG4 was not checked in this case.
The term IPT has been used to describe inflammatory and fibrosing tumoral processes of an undetermined cause that may involve a variety of organ system. It was first described in the lung as plasma cell granuloma.5 Although there is still no uniformly accepted classification system, IPT can be classified into three subtypes such as cellular type, fibrohistiocytic type, and lymphoplasmacytic type.6,7 Classical examples of cellular, fibrohistiocytic, and lymphoplasmacytic type were inflammatory myofibroblastic tumor (IMT), xanthogranulomatous inflammation, and IgG4-related IPT, respectively. While IMT is the possible neoplastic counterpart of IPT as it frequently shows genetic alteration (i.e., ALK translocation), lymphoplasmacytic or fibrohistiocytic type has much greater reactive aspects. Lymphoplasmacytic type, in particular, has close relations with IgG4-related sclerosing disease.6-8
The association between IPT and IgG4 was first reported in a patient with lymphoplasmocytic sclerosing pancreatitis9 accompanying a tumorous swelling in its head portion (i.e., pancreatic IPT).10 In recent years, extrapancreatic IPTs in the lung,8 liver,11 breast,6 stomach,12 ureter,13 and kidney14 also have been reported to be IgG4-related, and therefore they are now considered as one of IgG4-related disease. IgG4-related disease is a newly recognized fibroinflammatory condition characterized by IgG4-producing plasma cell expansion in affected organs and, often but not always, elevated serum IgG4 concentrations. Various symptoms and inflammatory conditions are noted depending on the affected organ. Previously recognized conditions such as retroperitoneal fibrosis, multifocal fibrosclerosis, Mikulicz's syndrome, Riedel's thyroiditis and IPT are now known to fall within the spectrum of this disease entity.15,16 Despite there are many reports on intraperitoneal IPTs including both cellular and lymphoplasmacytic type, only a few cases have been confirmed to be IgG4-related.1-4 The demonstrated cases were with a form of sclerosing mesenteritis2,3 of which margin is usually infiltrative or IPTs associated with other IgG4-related sclerosing disorders.1,4 By contrast, our case revealed a single well circumscribed intraperitoneal IgG4-related IPT.
Although IgG4-related IPT is a newly defined clinical entity, the mechanism by which IPT develops is still unclear. The exact role of IgG4 or IgG4-postivie plasma cells in this disease has not been elucidated. Only some clinical features such as hypergammaglobulineimia and hypocomplementemia supports an autoimmunity nature.11
For histological diagnosis, there should be 10 or more IgG4 positive lymphoplasmacytic cells per high power field.17 IMT should be in the list of differential diagnosis because of the similar microscopic features. Unlike IgG4-related IPT, IgG4 does not seem to play an important role in IMT. Instead, aberrant expression of ALK and related gene alteration implicates in the pathogenesis of IMT.18 In our case, immunohistochemical stains were negative for ALK, and therefore diagnosis of IMT was unlikely.
Although serum IgG4 level was not measured in our case, the diagnosis was not problematic because of the typical histologic findings. Actually, histologic finding is considered to be more important in the confirmatory diagnosis. For example, 15% of autoimmune pancreatitis was not associated with IgG4 elevation in a Korean study.19 Moreover, another report showed that serum IgG4 level did not increase in direct proportion with the concentration of IgG4 positive lymphoplasmacytic cells.20
In summary, we present a case of a single well circumscribed intraperitoneal IgG4-related IPT. The past H&E based diagnosis of IPT is being replaced by more accurate diagnoses such as IMT or IgG4-related IPT by simply adding immunohistochemistry with specific markers. For accurate diagnosis and appropriate therapy, it is important for both clinicians and pathologists to consider IgG4-related IPT when a single intraperitoneal mass is noticed.
Figures and Tables
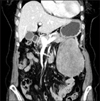 | Fig. 1CT with contrast enhancement. An 11×8 cm sized well defined heterogenous mass adjacent to the stomach was noted. |
 | Fig. 3Histologic features of the resected specimen. (A) The lymphoplasmacytic infiltrate showed the formation of germinal centers (H&E, ×200). High-power view showed an admixture of spindle-shaped cells and prominent lymphoplasma cells within fibrotic stroma (inset, H&E, ×400). (B) Immunohistochemical staining of IgG4. Up to 25/HPF IgG4 positive plasma cells were noted (×400). |
References
1. Sakemi R, So S, Morimitsu Y, et al. A case of IgG4-related sclerosing disorders involving the mesentery and the pancreas. Nihon Shokakibyo Gakkai Zasshi. 2011. 108:969–977.
2. Nomura Y, Naito Y, Eriguchi N, et al. A case of IgG4-related sclerosing mesenteritis. Pathol Res Pract. 2011. 207:518–521.
3. Chen TS, Montgomery EA. Are tumefactive lesions classified as sclerosing mesenteritis a subset of IgG4-related sclerosing disorders? J Clin Pathol. 2008. 61:1093–1097.
4. Masterson L, Del Pero MM, Donnelly N, Moffat DA, Rytina E. Immunoglobulin G4 related systemic sclerosing disease involving the temporal bone. J Laryngol Otol. 2010. 124:1106–1110.
5. Bahadori M, Liebow AA. Plasma cell granulomas of the lung. Cancer. 1973. 31:191–208.
6. Zen Y, Kasahara Y, Horita K, et al. Inflammatory pseudotumor of the breast in a patient with a high serum IgG4 level: histologic similarity to sclerosing pancreatitis. Am J Surg Pathol. 2005. 29:275–278.
7. Zen Y, Fujii T, Sato Y, Masuda S, Nakanuma Y. Pathological classification of hepatic inflammatory pseudotumor with respect to IgG4-related disease. Mod Pathol. 2007. 20:884–894.
8. Zen Y, Kitagawa S, Minato H, et al. IgG4-positive plasma cells in inflammatory pseudotumor (plasma cell granuloma) of the lung. Hum Pathol. 2005. 36:710–717.
9. Hamano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001. 344:732–738.
10. Adsay NV, Basturk O, Klimstra DS, Klöppel G. Pancreatic pseudotumors: non-neoplastic solid lesions of the pancreas that clinically mimic pancreas cancer. Semin Diagn Pathol. 2004. 21:260–267.
11. Zen Y, Harada K, Sasaki M, et al. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: do they belong to a spectrum of sclerosing pancreatitis? Am J Surg Pathol. 2004. 28:1193–1203.
12. Rollins KE, Mehta SP, O'Donovan M, Safranek PM. Gastric IgG4-related autoimmune fibrosclerosing pseudotumour: a novel location. ISRN Gastroenterol. 2011. 2011:873087.
13. Kim SA, Lee SR, Huh J, Shen SS, Ro JY. IgG4-associated inflammatory pseudotumor of ureter: clinicopathologic and immunohistochemical study of 3 cases. Hum Pathol. 2011. 42:1178–1184.
14. Shoji S, Nakano M, Usui Y. IgG4-related inflammatory pseudotumor of the kidney. Int J Urol. 2010. 17:389–390.
15. Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012. 366:539–551.
16. Masaki Y, Kurose N, Umehara H. IgG4-related disease: a novel lymphoproliferative disorder discovered and established in Japan in the 21st century. J Clin Exp Hematop. 2011. 51:13–20.
17. Chari ST, Smyrk TC, Levy MJ, et al. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006. 4:1010–1016.
18. Yamamoto H, Yamaguchi H, Aishima S, et al. Inflammatory myofibroblastic tumor versus IgG4-related sclerosing disease and inflammatory pseudotumor: a comparative clinicopathologic study. Am J Surg Pathol. 2009. 33:1330–1340.
19. Choi EK, Kim MH, Lee TY, et al. The sensitivity and specificity of serum immunoglobulin G and immunoglobulin G4 levels in the diagnosis of autoimmune chronic pancreatitis: Korean experience. Pancreas. 2007. 35:156–161.
20. Neild GH, Rodriguez-Justo M, Wall C, Connolly JO. Hyper-IgG4 disease: report and characterisation of a new disease. BMC Med. 2006. 4:23.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download