Abstract
Background/Aims
The purpose of this study was to estimate the prevalence of Barrett's esophagus (BE) and its association with reflux esophagitis (RE) and peptic ulcer disease detected by free charge endoscopy which was covered by the National Health Insurance at a secondary care hospital, and to compare the results of the biopsy of BE with that of cardiac intestinal metaplasia (CIM).
Methods
A total of 4,002 patients underwent endoscopy from March 2010 to December 2012. BE was diagnosed if there was histologically proven specialized intestinal metaplasia, and CIM was diagnosed if intestinal metaplasia was accompanied with chronic gastritis.
Results
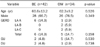
Four hundred twenty four patients underwent endoscopic biopsy, and the prevalence of BE was 1.0% (42/4,002). The mean age and the proportion of males in BE were significantly higher than those of the rest of study population, and BE had slight tendency related to RE than the rest of study population. CIM was observed in 34 patients and BE and CIM showed similar results, regarding age, sex and association with RE. The mean length of endoscopic Barrett's mucosa of BE group was 9.2±5.1 mm, and it was similar to that of CIM.
Conclusions
The prevalence of BE in the secondary care hospital was not low, and old age and male sex were significantly associated with BE. Because BE was observed in about 10% of biopsied patients and CIM was observed in a similar percentage with BE, the precise targeted biopsy is warranted and the biopsy method should be reestablished through the large prospective study of multiple secondary care hospitals.
Figures and Tables
Table 2
Comparison between the Barrett's Esophagus Group and the Cardiac Intestinal Metaplasia Group
References
1. Choi KS, Kwak MS, Lee HY, Jun JK, Hahm MI, Park EC. Screening for gastric cancer in Korea: population-based preferences for endoscopy versus upper gastrointestinal series. Cancer Epidemiol Biomarkers Prev. 2009. 18:1390–1398.
2. Ishimura N, Amano Y, Appelman HD, et al. Barrett's esophagus: endoscopic diagnosis. Ann N Y Acad Sci. 2011. 1232:53–75.
3. Chang CY, Cook MB, Lee YC, et al. Asian Barrett's Consortium. Current status of Barrett's esophagus research in Asia. J Gastroenterol Hepatol. 2011. 26:240–246.
4. Mueller J, Werner M, Stolte M. Barrett's esophagus: histopathologic definitions and diagnostic criteria. World J Surg. 2004. 28:148–154.
5. Liu GS, Gong J, Cheng P, Zhang J, Chang Y, Qiang L. Distinction between short-segment Barrett's esophageal and cardiac intestinal metaplasia. World J Gastroenterol. 2005. 11:6360–6365.
6. Miao Q, Peng YS, Cai GH, Chen XY. Comparison of intestinal metaplasia in gastric cardia and Barrett's esophagus. J Dig Dis. 2011. 12:272–278.
7. Voutilainen M, Juhola M, Färkkilä M, Sipponen P. Foveolar hyper-plasia at the gastric cardia: prevalence and associations. J Clin Pathol. 2002. 55:352–354.
8. Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999. 45:172–180.
9. Kim JH, Rhee PL, Lee JH, et al. Prevalence and risk factors of Barrett's esophagus in Korea. J Gastroenterol Hepatol. 2007. 22:908–912.
10. Kim JY, Kim YS, Jung MK, et al. Prevalence of Barrett's esophagus in Korea. J Gastroenterol Hepatol. 2005. 20:633–636.
11. Lee IS, Choi SC, Shim KN, et al. Prevalence of Barrett's esophagus remains low in the Korean population: nationwide cross-sectional prospective multicenter study. Dig Dis Sci. 2010. 55:1932–1939.
12. Lee JI, Park H, Jung HY, Rhee PL, Song CW, Choi MG. Prevalence of Barrett's esophagus in an urban Korean population: a multicenter study. J Gastroenterol. 2003. 38:23–27.
13. Park JJ, Kim JW, Kim HJ, et al. H. pylori and GERD Study Group of Korean College of Helicobacter and Upper Gastrointestinal Research. The prevalence of and risk factors for Barretts esophagus in a Korean population: A nationwide multicenter prospective study. J Clin Gastroenterol. 2009. 43:907–914.
14. Abdo-Francis JM, Sobrino-Cossío S, Bernal-Sahagún F, Hernández-Guerrero A. Prevalence of intestinal metaplasia of the gastric cardia and its relation with Helicobacter pylori strains cagA and vacA. Cir Cir. 2010. 78:315–321.
15. Bak YT, Jung GM, Yeon JE, et al. Validity of the specialized columnar epithelium as a diagnostic criterion of the short segment Barrett's esophagus. Korean J Intern Med. 1998. 13:99–103.
16. Lee JS, Kim HW, Lee JH, Youn HS, Jung WT, Ko GH. Relationships between types of proximal gastric mucosa and clinicopathological features. Korean J Pathol. 2003. 37:15–18.
17. Kim CW, Lee BI, Kim BW, et al. Immunohistochemical expression of the p53 and Ki-67 proteins in Barrett's esophagus in Korea. Korean J Gastroenterol. 2005. 46:189–195.
18. Ormsby AH, Vaezi MF, Richter JE, et al. Cytokeratin immunoreactivity patterns in the diagnosis of short-segment Barrett's esophagus. Gastroenterology. 2000. 119:683–690.
19. Rothery GA, Patterson JE, Stoddard CJ, Day DW. Histological and histochemical changes in the columnar lined (Barrett's) oesophagus. Gut. 1986. 27:1062–1068.
20. Chung JW, Lee GH, Choi KD, et al. Prevalence of the endoscopic Barrett's esophagus determined by palisading vessel and inter-observer variation. Korean J Gastrointest Endosc. 2007. 34:239–243.
21. Chandrasoma P, Wickramasinghe K, Ma Y, DeMeester T. Is intestinal metaplasia a necessary precursor lesion for adenocarcinomas of the distal esophagus, gastroesophageal junction and gastric cardia? Dis Esophagus. 2007. 20:36–41.
22. Chandrasoma P, Wijetunge S, DeMeester S, et al. Columnar-lined esophagus without intestinal metaplasia has no proven risk of adenocarcinoma. Am J Surg Pathol. 2012. 36:1–7.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download