Abstract
Systemic sclerosis (SSc) is a chronic systemic disease that affects the skin, lungs, heart, gastrointestinal tract, kidneys, and musculoskeletal system. Although up to 90% of patients with scleroderma have been estimated to have gastrointestinal involvement, liver disease has been reported only rarely. A 51-year-old woman was hospitalized due to esophageal variceal bleeding. Her serum was positive for anti-nuclear antibody and anti-centromere antibody. Sclerodactyly was noted on both hands, and she had recently developed Raynaud's syndrome. Punch biopsy of the hand showed hyperkeratosis, regular acanthosis, and increased basal pigmentation in the epidermis, and thick pale collagenous bundles in the dermis. Liver biopsy showed chronic active hepatitis with bridging fibrosis. Consequently, she was diagnosed with liver cirrhosis due to autoimmune hepatitis (AIH) combined with SSc. AIH had subsided after administration of prednisolone at 40 mg per day. She received 5-10 mg/day of prednisolone as an outpatient, and her condition has remained stable. Patients with either AIH or SSc should be monitored for further development of concurrent autoimmune diseases. The early diagnosis of AIH combined with SSc will be helpful in achieving optimal management.
Autoimmune hepatitis (AIH) is a generally progressive, chronic disease with occasionally fluctuating activity that occurs worldwide in children and adults.1 Although the cause of AIH is unknown, aberrant autoreactivity is thought to play a role in its pathogenesis.2 AIH has been reported in patients with various diseases, including type 1 diabetes, thyroiditis, glomerulonephritis, autoimmune hemolytic anemia, autoimmune thrombocytopenia, and ulcerative colitis.3-5 However, systemic connective tissue diseases, such as systemic lupus erythematosus, undifferentiated connective tissue disease, mixed connective tissue disease, and systemic sclerosis (SSc), have been reported infrequently in association with AIH.6 Here, we report a patient presenting with liver cirrhosis due to AIH combined with SSc, and present a review of the relevant literature.
A 51-year-old woman visited our hospital with hematemesis. She had no other relevant medical history and no alcohol consumption and not been taking any medication. The patient's blood pressure was 100/60 mmHg and her pulse was 100 beats/min. There was no icteric sclera. Splenomegaly was present. Livedo reticularis and telangiectasia of the extremities were observed. Sclerodactyly was seen on the hands (Fig. 1A). The baseline laboratory tests showed pancytopenia and liver function tests revealed albumin 3.8 g/dL, total bilirubin 1.4 mg/dL, AST 124 U/L and ALT 54 U/L. The total protein was 6.9 g/dL and the globulin was 3.0 g/dL. The prothrombin time INR was 1.3. Viral serologies (hepatitis B surface antigen, anti-hepatitis B surface antigen, immunoglobulin G anti-hepatitis B core antigen, anti-hepatitis C virus, cytomegalovirus, Epstein-Barr virus, herpes simplex virus, human immunodeficiency virus) were all negative. Immunological tests revealed a discrete speckled pattern and a homogeneous pattern of fluorescent antinuclear antibody (FANA) at 2,560× and ELISA were also elevated, to 260 units (normal <20 units) (Table 1). Neither shifting dullness and fluid wave in abdominal physical examination nor encephalopathy was observed. Liver function was evaluated as grade A, according to the Child-Pugh classification. Abdominal ultrasonography revealed liver cirrhosis with splenomegaly and a moderate amount of ascites. We performed paracentesis and serum ascites albumin gradient was 2.5. Endoscopy showed grade 4 varices (F3), and a small ulcer was detected in one of the varices, which was suggestive of hemorrhage. We performed band ligation of the esophageal varices. We could not find other cause of portal hypertension. Liver biopsy showed partial expansion with lymphoplasmacytic infiltration, bridging fibrosis, and multifocal drop out of hepatocytes, replaced by lymphoid cells in the lobules, suggesting chronic active hepatitis (Fig. 2). These histological and clinical findings were consistent with AIH. The International Autoimmune Hepatitis Group score was 18. Additionally, the patient had suffered from Raynaud's syndrome, sclerodactyly, arthralgia, and fatigue for 3 years prior to presentation. From 2 months prior to presentation, she had frequent episodes of Raynaud's phenomenon and aggravated sclerodactyly. Punch biopsy was performed at the dorsum of the left hand, and the dermis was shown to have thick and pale collagenous bundles with rare fibroblasts and inflammatory cells (Fig. 1B), suggestive of morphea or SSc. There was no abnormal finding on echocardiography. High resolution CT was performed and there was no evidence of interstitial pulmonary fibrosis. Esophageal varices were eradicated after four sessions of prophylactic esophageal variceal ligation and there was no recurrent variceal hemorrhage. Raynaud's phenomenon was treated with calcium channel blockers and is currently well controlled. AIH subsided after administration of prednisolone at a dose of 40 mg/day during 2 weeks. The patient has since received 5-10 mg/day of prednisolone as an outpatient, and her condition has remained stable.
SSc is a chronic systemic disease that affects the skin, lungs, heart, gastrointestinal tract, kidneys, and musculoskeletal system. The disorder is characterized by three features: tissue fibrosis, vessel vasculopathy, and an autoimmune response associated with specific autoantibodies.7 Although up to 90% of patients with SSc have been estimated to have gastrointestinal involvement,8 liver disease has been reported only rarely in these cases. In a review of 727 patients with scleroderma, only eight (1.1%) had hepatic involvement.9 Primary biliary cirrhosis (PBC) is the most frequently encountered liver disease in SSc patients. In a series of 83 patients with primary biliary cirrhosis, 17% of patients had concomitant SSc.10 The pathological mechanisms of AIH in patients with SSc remain unclear. However, AIH may be due, in part, to dysfunction of both cellular and humoral immunity related to SSc, as anti-centromere antibody has been detected in up to 13% of patients with AIH.11-13 There have been only two reports in the literature regarding investigations related to anti-centromere antibodies in patients with AIH, which indicated the presence of these antibodies at a low frequency of 3% in cases of AIH.14,15 In the Japanese population, the frequency of the discrete speckled pattern of FANA (closely associated with anti-centromere antibody) is increased in AIH patients with anti-centromere antibody, supporting the close association between the AIH and SSc. However, no definite conclusions can be drawn from these findings, and further studies are necessary to examine the relationship between anti-centromere antibody and AIH.
The diagnosis of AIH in this case was reasonable because the patient with SSc fulfilled all the diagnostic criteria of the International Autoimmune Hepatitis Group for AIH:16 1) increased AST and ALT levels with ALP levels less than three times the normal value; 2) total γ-globulin or IgG levels more than 1.5 times the upper limit of normal; 3) high titers of ANA (>1 : 80); 4) characteristic histological liver damage (i.e., interface or periportal hepatitis; histological evidence of damage associated with PBC was absent, which excluded primary biliary cirrhosis/AIH overlap); 5) negative for all viral serology (hepatitis A, B, and C, cytomegalovirus, Epstein-Barr virus); and 6) there was no evidence of other causes of liver disorders. Four cases of AIH exclusive of PBC in patients with SSc have been reported to date in the literature (Table 2). Among them, three cases were diagnosed with SSc 1 month to 7 years before developing AIH, and in the remaining one case, SSc and AIH were diagnosed together. This case also had SSc and AIH together at the time of initial diagnosis. These results indicate that patients with SSc may be at increased risk of developing AIH, and that patients with AIH also may have possibility for risk of developing SSc. In this case, there was liver cirrhosis due to AIH combined with SSc, which was much further advanced liver disease than the four reported cases. This case also was a limited SSc that did not show calcinosis cutis, Raynaud's phenomenon, esophageal dysfunction, sclerodactyly, and telangiectasia (CREST) or calcinosis cutis, Raynaud's phenomenon, sclerodactyly, telangiectasia syndrome (CRST) shown previously in reported four cases. Treatment of AIH is difficult in patients with SSc, as patients (primarily with diffuse cutaneous SSc) receiving more than 15 mg prednisolone daily are at high risk of renal crisis related to their SSc.17 However, in all five cases after high dose prednisolone (30-40 mg), symptoms were improved and the outcome of AIH was favorable. However, further studies in larger numbers of cases are required to determine the treatment outcome of combined AIH and SSc. The combination of SSc and AIH in the early phase may be overlooked if not examined by a specialist in rheumatology. The symptoms may appear gradually during the clinical course, and therefore it is important to consider the combination of SSc and AIH even in cases with a confirmed diagnosis of either disease entity. The results of the present case indicate that it is necessary to consider the possibility of AIH even-in patients with limited SSc presenting with skin manifestations, such as Raynaud's phenomenon and sclerodactyly.
In conclusion, patients with either AIH or SSc should be monitored for further development of concurrent autoimmune disease. The early diagnosis of AIH combined with SSc will be helpful in achieving optimal management in such cases.
Figures and Tables
 | Fig. 1Skin manifestations and biopsy. (A) Sclerodactyly development at the tips of the fingers, skin indurations, and fixed flexion contractures at the proximal interphalangeal joints. (B) Section of the skin biopsy specimen showing collagen bundles of the reticular dermis appear to be thickened, hypocellular and deeply eosinophilic (H&E, ×200). |
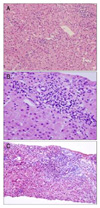 | Fig. 2Liver biopsy. (A) Multiple drop out of hepatocytes replaced by lymphoid cells and acidophilic bodies were seen in the acini (H&E, ×200). (B) Portal and periportal infiltration of lymphoid cells and a few plasma cells were seen. The bile ducts seemed to be intact (H&E, ×400). (C) Masson's trichrome staining revealed portal and periportal fibrosis with bridging (×100). |
Table 2
Literature Reviews of Autoimmune Hepatitis (AIH) Combined with Systemic Sclerosis

CREST, calcinosis, Raynaud's phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia syndrome; CRST, calcinosis cutis, Raynaud's phenomenon, sclerodactyly, telangiectasia syndrome; Raynaud's, Raynaud's phenomenon; F, female; FANA, fluorescent anti-nuclear antibody; Anti-centromere, anti-centromere antibody; PDL, prednisolone; SSc, systemic sclerosis.
References
1. Czaja AJ, Souto EO, Bittencourt PL, et al. Clinical distinctions and pathogenic implications of type 1 autoimmune hepatitis in Brazil and the United States. J Hepatol. 2002. 37:302–308.
2. Krawitt EL. Autoimmune hepatitis. N Engl J Med. 2006. 354:54–66.
3. McFarlane IG. The relationship between autoimmune markers and different clinical syndromes in autoimmune hepatitis. Gut. 1998. 42:599–602.
4. Homberg JC, Abuaf N, Bernard O, et al. Chronic active hepatitis associated with antiliver/kidney microsome antibody type 1: a second type of "autoimmune" hepatitis. Hepatology. 1987. 7:1333–1339.
5. Gregorio GV, Portmann B, Reid F, et al. Autoimmune hepatitis in childhood: a 20-year experience. Hepatology. 1997. 25:541–547.
6. West M, Jasin HE, Medhekar S. The development of connective tissue diseases in patients with autoimmune hepatitis: a case series. Semin Arthritis Rheum. 2006. 35:344–348.
7. Youssef WI, Tavill AS. Connective tissue diseases and the liver. J Clin Gastroenterol. 2002. 35:345–349.
8. Cohen S. The gastrointestinal manifestations of scleroderma: pathogenesis and management. Gastroenterology. 1980. 79:155–166.
9. Bartholomew LG, Cain JC, Winkelmann RK, Baggenstoss AH. Chronic disease of the liver associated with systemic scleroderma. Am J Dig Dis. 1964. 9:43–55.
10. Clarke AK, Galbraith RM, Hamilton EB, Williams R. Rheumatic disorders in primary biliary cirrhosis. Ann Rheum Dis. 1978. 37:42–47.
11. Ishikawa M, Okada J, Shibuya A, Kondo H. CRST syndrome (calcinosis cutis, Raynaud's phenomenon, sclerodactyly, and telangiectasia) associated with autoimmune hepatitis. Intern Med. 1995. 34:6–9.
12. Yabe H, Noma K, Tada N, Mochizuki S, Nagano M. A case of CREST syndrome with rapidly progressive liver damage. Intern Med. 1992. 31:69–73.
13. Bernstein RM, Callender ME, Neuberger JM, Hughes GR, Williams R. Anticentromere antibody in primary biliary cirrhosis. Ann Rheum Dis. 1982. 41:612–614.
14. Cassani F, Bianchi FB, Lenzi M, Volta U, Pisi E. Immunomorphological characterisation of antinuclear antibodies in chronic liver disease. J Clin Pathol. 1985. 38:801–805.
15. Makinen D, Fritzler M, Davis P, Sherlock S. Anticentromere antibodies in primary biliary cirrhosis. Arthritis Rheum. 1983. 26:914–917.
16. Johnson PJ, McFarlane IG. Meeting report: International Autoimmune Hepatitis Group. Hepatology. 1993. 18:998–1005.
17. Steen VD, Medsger TA Jr. Case-control study of corticosteroids and other drugs that either precipitate or protect from the development of scleroderma renal crisis. Arthritis Rheum. 1998. 41:1613–1619.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download