Abstract
Mucosa-associated lymphoid tissue (MALT) lymphoma is a typical primary gastrointestinal lymphoma, particularly in the stomach. Although primary rectal lymphoma is rare, it may present as a subepithelial tumor. Several techniques have been proposed for a tissue diagnosis in subepithelial tumor, including endoscopic ultrasonography (EUS)-guided fine needle aspiration (EUS-FNA), EUS-guided trucut biopsy (EUS-TCB), and tacked biopsy. However the diagnostic efficacy of these techniques appears to be limited. The unroofing technique involves removal of the overlying mucosa, thereby exposing the subepithelial lesion. It was originally reported as a method for endoscopic treatment of colorectal lymphangioma. In this case, a subepithelial tumor of the rectum was diagnosed using the endoscopic unroofing technique. This is a useful modality for the diagnosis of subepithelial tumor, because it provides histologic results in a safe and rapid manner.
The diagnostic evaluation and subsequent management of subepithelial masses varies considerably because approaches to these lesions are still evolving.1 EUS can reliably differentiate subepithelial tumor.1,2 If a subepithelial lesion is found to be a hypoechoic mass in the third or fourth echo layer on EUS examination, then tissue sampling should be considered to establish the diagnosis.1 Several techniques have been proposed for a tissue diagnosis, including EUS-guided fine needle aspiration (EUS-FNA), EUS-guided trucut biopsy (EUS-TCB), and tacked biopsy. However, the diagnostic efficacy of these techniques appears to be limited.1,2
The unroofing technique involves removal of the overlying mucosa, thereby exposing the subepithelial lesion. This technique was originally reported as a method for the endoscopic treatment of colorectal lymphangioma.2 We report a case of mucosa-associated lymphoid tissue (MALT) lymphoma which appeared as a form of subepithelial rectal tumor and was diagnosed with the unroofing technique.
A 44 year-old man was referred with a subepithelial tumor of the rectum, which was detected at another hospital. He had complained of a hematochezia for 2 months. However, body weight loss, fever, chills and abdominal pains were absent. Beginning 8 years earlier, the patient took medication for diabetes mellitus, Parkinson's disease, and gout. His vital signs were stable; blood pressure 100/60 mmHg, heart rate 88 beats/minute, respiratory rate 20 breaths/minute, and body temperature 36.8℃. There was no lymphadenopathy and, abdominal rebound tenderness. Bowel sounds were normal.
His laboratory data were as follows; white blood cells 7,710/mm3 (neutrophil 61.2%, lymphocyte 29.3%, monocyte 6.3%, eosinophil 1.0%), hemoglobin 14.1 g/dL, platelets 415,000/mm3, Na 139 mEq/L, K 4.7 mEq/L, Cl 102 mEq/L, BUN 10.2 mg/dL, creatinine 0.8 mg/dL, AST 20 IU/L, ALT 29 IU/L, total bilirubin 1.5 mg/dL, ALP 76 IU/L, total protein 7.7 g/dL, albumin 4.7 g/dL. CEA levels were within the normal range. A simple chest X-ray and chest CT revealed no abnormalities. Abdominopelvic CT and MRI showed a round intraluminal polypoid mass in the right lateral wall of the distal rectum (6.5×3.3 cm) (Fig. 1). Small lymph nodes were observed in the perirectal and presacral area.
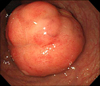
On colonoscopy, there was a protruding 3.3 cm subepithelial tumor in the rectum 2 cm above the anal verge (Fig. 2). EUS revealed the lesion to be a hypoechoic mass originating from the fourth endosonographic layer (Fig. 3). Using a flex knife, the center of the subepithelial lesion was targeted. Direct biopsies were obtained, after removal of the overlying mucosa (Fig. 4). Pathologic evaluation of the specimens revealed diffuse monotonous infiltration of small lymphoid cells with a squeezing artifact (Fig. 5A, B). Based on immunohistochemistry, lymphoma cells were positive for CD 20 and BCL-2, but negative for cyclin D1, CD 10, and BCL-6. These findings were compatible with marginal zone B-cell lymphoma of MALT (Fig. 5C, D). An immunoglobulin heavy chain gene rearrangement study was not performed. The patient was referred to hemato-oncology and treated with 4 cycles of cyclophosphamide, hydroxydaunomycin, oncovin, prednisolone (CHOP) chemotherapy and regional radiation therapy. Follow-up colonoscopy after 11 months showed completely healed lesion without remnants.
In this case, a subepithelial tumor of the rectum was diagnosed using the endoscopic unroofing technique. This is a useful modaliaty to diagnose subepithelial tumor, because it provides histologic results rapidly and safely.2 However, there are few reports of the unroofing technique in the literature.
Primary rectal lymphoma is rare and accounts for only ~1% of all colorectal malignancies and 0.1% of rectal primary tumor.3,4 However, almost 60% of primary rectal tumor is MALT lymphoma.5,6
In 1983, Isaacson and Wright7 introduced the concept of MALT lymphoma. MALT lymphoma is described as a subtype of non-Hodgkin's lymphoma derived from marginal-zone lymphocytes.8,9 Most MALT lymphomas arise from the gastrointestinal organs.10 However, it is able to develop in diverse anatomic locations such as the salivary gland, thyroid, lung and breast.9,10
A relationship between gastric MALT lymphoma and Helicobacter pylori (H. pylori) infection has been clearly established, and low grade gastric MALT lymphoma is known to regress after eradication of H. pylori.9 However, it is unclear if colorectal MALT lymphoma is related to H. pylori infection.10 According to previous report, the majority of patients with rectal MALT lymphoma underwent surgical or endoscopic resection as a cure.10 In the present case, H. pylori infection was not confirmed. Thus, the eradication of H. pylori was not attempted.
While gastric MALT lymphoma includes various characteristics, such as ulcer or erosion, MALT lymphoma of the rectum shows mostly, elevated lesions, such as polyps or subepithelial tumors.10 EUS could be applied to the diagnosis and recognition of subepithelial tumor, but it is possible that even small benign subepithelial tumors suspected on EUS would be reported as malignancy after resection.1,2 Particularly in the case of lymphoma, a definitive diagnosis is often reached by histological confirmation, since this is crucial for treatment and prognosis.
EUS can evaluate and differentiate subepithelial tumor, but when performed alone is not sufficient for an accurate diagnosis.2 EUS-FNA is commonly used to confirm the presence of malignancy, but limited by the decreased feasibility of FNA for smaller lesions and often fails to provide sufficient tissue for immunohistochemistry.1 Previous reports have shown inconsistent diagnostic yields (38-82%).2 Endoscopic resection is also used for diagnosis, but the clinical usefulness of endoscopic resection of the entire lesion is unclear.2
The unroofing technique was originally reported as a method for endoscopic treatment of colorectal lymphangioma.2,11 Like subepithelial tumors, colorectal lymphangiomas are widely attached to the colonic wall.11 Therefore, snare polypectomy of a colonic lymphangioma may lead to perforation.11 Thus, early studies performed endoscopic resection of the upper half of the lymphangioma, which is called unroofing.11 There are some scattered reports of the unroofing technique for endoscopic resection of upper gastrointestinal subepithelial tumors, such as esophageal submucosal tumors, symptomatic duodenal lipomas, and duodenal duplication cyst.2
The unroofing technique provides excellent tissue yields allowing for immunohistochemistry and an improved diagnostic yield (>90%).2,12 Sufficient tissue samples allow for complete pathological examination of the tumors, including architectural details, immunohistochemical staining, and determination of the mitotic index.2 In addition, the mean procedure time is relatively short (~10 minutes).2 Major complications, although rare, include perforations and massive bleeding that require blood transfusion or surgical intervention.2 Minor bleeding can be successfully controlled by endoscopic hemostasis.2
The unroofing technique is not commonly used, however, given the above findings, it can be used to diagnose the subepithelial tumor. In conclusion, the unroofing technique may be a one feasible option to make a diagnosis for the rectal epithelial tumor.
Figures and Tables
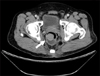 | Fig. 1Abdominopelvic computed tomography. A 6.5×3.3 cm intraluminal polypoid mass in the right lateral wall of the distal rectum was noted (arrow). |
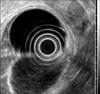 | Fig. 3Endoscopic ultrasonography finding. A hypoechoic mass originating from the fourth endosonographic layer was noted. |
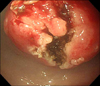 | Fig. 4Unroofing technique. Using a flex knife, direct biopsies were obtained after removing the overlying mucosa. |
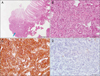 | Fig. 5(A) Low power view showed focal diffuse lymphoid infiltration (arrow; H&E, ×40). (B) Dense infiltration of centrocyte-like small lymphoid cells (H&E, ×400). (C) Lymphoid cells positive for CD20 by immunohistochemical staining (×400). (D) Lymphoid cells negative for cyclin D1 by immunohistochemical staining (×400). |
References
1. Hwang JH, Rulyak SD, Kimmey MB. American Gastroenterological Association Institute. American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology. 2006. 130:2217–2228.
2. Lee CK, Chung IK, Lee SH, et al. Endoscopic partial resection with the unroofing technique for reliable tissue diagnosis of upper GI subepithelial tumors originating from the muscularis propria on EUS (with video). Gastrointest Endosc. 2010. 71:188–194.
3. Yamamoto R, Kato S, Shimazaki K, et al. A case of primary rectal mucosa-associated lymphoid tissue lymphoma treated by endoscopic mucosal resection. Digestive Endoscopy. 2005. 17:172–174.
4. Gavioli M, Bagni A, Santacroce G, Piccagli I, Natalini G. Endorectal sonographic appearances of rectal MALT lymphoma, its response to therapy, and local recurrence. J Clin Ultrasound. 2001. 29:401–405.
5. Yatabe Y, Nakamura S, Nakamura T, et al. Multiple polypoid lesions of primary mucosa-associated lymphoid-tissue lymphoma of colon. Histopathology. 1998. 32:116–125.
6. Tanaka S, Ohta T, Kaji E, Kosaka T, Murakami I. EMR of mucosa-associated lymphoid tissue lymphoma of the rectum. Gastrointest Endosc. 2003. 57:956–959.
7. Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. 1983. 52:1410–1416.
8. Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994. 84:1361–1392.
9. Takenaka R, Tomoda J, Sakata T, et al. Mucosa-associated lymphoid tissue lymphoma of the rectum that regressed spontaneously. J Gastroenterol Hepatol. 2000. 15:331–335.
10. Ahlawat S, Kanber Y, Charabaty-Pishvaian A, et al. Primary mucosa-associated lymphoid tissue (MALT) lymphoma occurring in the rectum: a case report and review of the literature. South Med J. 2006. 99:1378–1384.
11. Mimura T, Kuramoto S, Hashimoto M, et al. Unroofing for lymphangioma of the large intestine: a new approach to endoscopic treatment. Gastrointest Endosc. 1997. 46:259–263.
12. de la Serna-Higuera C, Pérez-Miranda M, Díez-Redondo P, et al. EUS-guided single-incision needle-knife biopsy: description and results of a new method for tissue sampling of subepithelial GI tumors (with video). Gastrointest Endosc. 2011. 74:672–676.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download