Abstract
Background/Aims
There was a spiking incidence of acute hepatitis A (AHA) in 2009 summer, but it went down drastically after an outbreak of influenza A (H1N1). We assessed the relationship between 2009 H1N1 pandemic and AHA prevalence from August to December 2009.
Methods
We compared AHA cases nationwide and in our hospital for the period from the latter half of 2008 to the end of 2010. H1N1 cases in our hospital from August 2009 to December 2009 were included in the study and the correlation between 2009 H1N1 pandemic and AHA prevalence was assessed.
Results
The national surveillance system reported 2,233, 7,895, 15,231 and 7,660 AHA cases from 2007 to 2010, respectively. A similar trend was noted in our hospital in the same periods. Although the national total incidence was increased in 2009, it showed steep decreasing trend line in the final 21 weeks of 2009 (weeks 32-52), as compared with 2008 and 2010. The mean weekly incidence percentage (AHA cases in a week/total in a year) in weeks 32-52 of 2009 was 1.17±0.55%, significantly lower than that in 2008 and 2010 (1.61±0.43% and 1.56±0.51%; p<0.001). Furthermore, we found a significant negative correlation between 2009 H1N1 pandemic and AHA in our hospital for weeks 32-52 of 2009 (r=-0.597; p<0.001).
Commonly called 'swine flu,' novel influenza A (H1N1) virus (2009 H1N1) is a hybrid virus composed of porcine, avian, and human genes. This virus is transmitted by droplet and potentially by touching contaminated surfaces or the hands of infected individuals.1 After early outbreaks in North America in April 2009, 2009 H1N1 rapidly spread worldwide and was declared a global pandemic in June of that year.2 In Korea, the first 2009 H1N1 patient was reported on May 2, 2009;3 since then, the number of infections has increased continually.
On the other hand, hepatitis A virus (HAV) is a positive-stranded RNA virus in the family Picornaviridae and is transmitted by the fecal-oral route.4 Acute hepatitis A (AHA) is a major public health problem in both developed and developing nations. Korea is no exception and has been confronted with a community-wide AHA outbreak in recent years.5,6
Since August 2009, interestingly the incidence of AHA seemed to be in inverse proportion to that of 2009 H1N1 pandemic has been occurred in our hospital as tertiary referral center. On the other hand, though in 2007, 2008, 2010, there was also decreased incidence of AHA after September, it was gradually going down much more. Thus, we intended to find out the factors associated with declining incidence of AHA and assessed the possible relationship between 2009 H1N1 pandemic and the prevalence of AHA from August to December 2009 in the Republic of Korea.
H1N1-positive cases from August to December 2009 in Soon Chun Hyang University Bucheon Hospital (SCH hospital; Bucheon, Korea) were included in the study. We performed nasopharyngeal washing on all patients with suspected 2009 H1N1 infection who had acute febrile respiratory illness (cough, rhinorrhea, sore throat, or fever). 2009 H1N1 infection was confirmed by real-time rRT-PCR of nasopharyngeal specimens. All confirmed patients were provided with oseltamivir.
Data from sentinel cases reported to the Korea Centers for Disease Control and Prevention (KCDC) and medical records of SCH hospital were used for analysis of HAV infections during 2007-2009. We received informed consent for use of medical records from all AHA patients in SCH hospital.
HAV infection was defined as the presence of both anti-HAV IgM in serum and specific clinical symptoms. Microparticle capture enzyme immunoassay was used for detection of serum IgM.
Data were expressed as means, standard deviations, and percentage, as appropriate. The correlation between the prevalence of 2009 H1N1 and HAV infection was examined from the latter half of 2008 to the end of 2010. To compare the mean weekly incidence in each year, t-test was used. A Pearson test was used for correlation between incidence of AHA and that of 2009 H1N1. Data were analyzed using the SPSS software package (version 12.0; SPSS Inc., Chicago, IL, USA). A p-value of <0.05 was considered to be statistically significant.
According to the KCDC, more than 700,000 cases of 2009 H1N1 and 187 related deaths were reported in Korea during 2009. rRT-PCR was performed at the SCH hospital on a total of 11,083 specimens during 2009, of which 5,342 were positive. Of these, 284 patients were hospitalized. There were no fatalities. Of the positive patients, 2,873 were male (53.8%), and the median age was 15.7 years (range, 0-83 years). Approximately 80% of patients were <20 years old, and ~1% were >60 years old (Fig. 1). The weekly incidence of 2009 H1N1 at the SCH hospital in the latter half of 2009 is shown in Fig. 2.
The incidence of AHA in Korea is increasing at an alarming rate in recent years. 2,233, 7,895, 15,231 and 7,660 AHA cases were reported from 2007 to 2010, respectively by the national surveillance system. A similar trend was noted at the SCH hospital in the same years (Fig. 3).
Of the AHA 477 patients at the SCH hospital, 291 were male (61%) with a median age of 40 years (range, 15-84 years). The peak incidence was in those aged 30-39 (Fig. 4). In most patients, the infection resolved without complications, and they were discharged. However, seven patients needed hemodialysis due to acute renal failure, and two of these received molecular adsorbent recirculating system due to combined fulminant hepatic failure. Although there were no fatalities, one patient was referred for liver transplantation.
Although the total number of HAV infections was increased, during weeks 32-52 of 2009, the mean weekly incidence percentage (AHA cases in a week/total in a year) was significantly lower than that during the same period in 2008 and 2010 (1.17±0.55% vs. 1.61±0.43% and 1.56±0.51%; p<0.001; Fig. 5). Furthermore, a significant negative correlation between 2009 H1N1 pandemic and AHA was detected in our hospital during surveillance weeks 32-52 in 2009 (r=-0.597; p<0.05; Fig. 6).
HAV infection is transmitted by the fecal-oral route and is closely associated with poor personal hygiene and inadequate sanitation.7 To reduce the spread of HAV, the World Health Organization (WHO) emphasizes the importance of "adequate supplies of safe-drinking water and proper disposal of sewage within communities, combined with personal hygiene practices, such as regular hand-washing".8
Until about 30 years ago, most adults in Korea had protective antibodies to HAV through natural infection during childhood. However, owing to socioeconomic development and improved sanitation, the seroprevalence of anti-HAV antibodies decreased dramatically during the last 20-30 years.5,6
In Korea, AHA was legally designated a communicable disease in 2000, and the national surveillance system started in 2001. Since that time, the number of AHA cases has increased continually.9 As a result, Korea is currently faced with a nationwide AHA outbreak. However, interestingly in our hospital, the incidence of AHA seemed to decrease during and after an outbreak of 2009 H1N1. Our data showed that the incidence of AHA did indeed decrease during the 2009 H1N1 pandemic.
To our knowledge, not much is known about the relationship between HAV and 2009 H1N1. And there is also little evidence that the 2009 H1N1 has a direct interruption on infectivity of HAV. However, we are under the impression that efforts to contain the spread of 2009 H1N1 also had an effect on HAV transmission.
Similar to other influenza viruses, the 2009 H1N1 is spread from person to person via droplet or by contact with contaminated hands or surfaces.10 The 2009 H1N1 pandemic emphasized the importance of public health measures in reducing transmission of the virus before introduction of vaccination. The WHO has recommended that "people who are ill should cover their mouth and nose when coughing or sneezing, stay home when they are unwell, clean their hands regularly and keep some distance from healthy people, as much as possible".2 Even in Korea, the government and the population tried to reduce 2009 H1N1 transmission by improving health care and personal hygiene, such as hand washing and wearing masks. We believe that such efforts also interrupted HAV transmission in the latter half of 2009.
On the other hand, another important method to be specially considered for controlling HAV infection of Korea, is active immunoprophylaxis by vaccination. Hepatitis A vaccine was first developed in the mid-1990s; four purified, formalin-inactivated vaccines are currently available.11 A single-dose vaccine is protective for 10 years in more than 94% of cases.12,13 Despite this efficacy, only Israel routinely vaccinates children, following a universal immunization program initiated in 1999. AHA incidence in Israel decreased from 50.4 to 2.2-2.5 per 100,000 during 1993-1998 and 2002-2004, respectively. AHA outbreaks in Israel were essentially eliminated.14,15
In contrast, vaccination in Korea was only recommended for persons at risk, i.e., those traveling to hepatitis A-endemic nations, men who have sex with men, persons with clotting-factor disorders, persons with chronic liver disease, etc.6 Considering that the incidence of HAV infection in Korea stands at 27.4 cases per 100,000, a childhood vaccination program would be an effective method of reducing AHA-related morbidity and mortality.
Since the beginning of 2010, the incidence of 2009 H1N1 in Korea has decreased dramatically. The incidence of AHA has also dropped somewhat but remain high. According to the KCDC, AHA will fortunately be included in the routine childhood vaccination program soon. Together with forthcoming routine vaccination, if good personal hygiene habits (for example, regular hand washing) continues to be practiced, we believe that AHA outbreaks will be eliminated from Korea in the near future.
Our study has some limitations. First, we cannot be sure whether data of KCDC and SCH represent real incidence of AHA and 2009 H1N1 infection. Because we included only symptomatic patients requiring hospital visit, and other atypical cases of AHA16 or 2009 H1N1 patients that have mild symptoms may be not included. Second, it is well known that the diagnosis of other acute febrile diseases (for example, liver abscess, acute appendicitis, tsutsugamushi disease, etc.) may be delayed when pandemic influenza. In the latter half of 2009, likewise, there may be AHA patients with delayed diagnosis or undiagnosed patients. Finally, in this study, we tried to look for the other factors such as total doses of HAV vaccination, the prevalence of HAV IgG, the power of advertisement for protection of AHA and so on, but the data could not represent the possible causes. For example, the people who was inoculated against HAV was about 0.36 million in 2007, 0.47 million in 2008, 0.53 million in 2009 and 1.06 million in 2010. However, in the first half of 2009, the incidence of AHA was rapidly increased compared to that of 2008. On the other hand, the incidence of AHA in the latter half of 2009 was comparable to that of 2010. Here, we just showed a correlation between 2009 H1N1 pandemic and the prevalence of AHA in this study, but its causal relationship has not been conclusively proven.
In conclusion, despite the limitations of the study and no direct evidence, our data suggested that measures taken against the 2009 H1N1 pandemic might be associated with reducing HAV transmission.
Figures and Tables
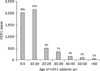 | Fig. 1The incidence of novel influenza A (H1N1) by age at the Soon Chun Hyang University Bucheon Hospital during 2009. Approximately 80% of patients were <20 years old, and ~1% were >60 years old. |
 | Fig. 2Number of influenza A (H1N1)-positive patients at the Soon Chun Hyang University Bucheon Hospital in weeks 32-52 of 2009. The peak incidence was at weeks 43-44 (October) of 2009, and it has been falling since then. |
 | Fig. 3Acute hepatitis A (AHA) cases in Korea nationwide (A) and in the Soon Chun Hyang University Bucheon Hospital (B) during 2007-2010. The incidence of AHA in Korea has increased at an alarming rate in recent years (A). A similar trend was noted at the hospital in the same years (B). |
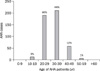 | Fig. 4The incidence of acute hepatitis A (AHA) by age at the Soon Chun Hyang University Bucheon Hospital during 2009. The peak incidence was in those aged 30-39 years. |
 | Fig. 5Weekly acute hepatitis A (AHA) incidence percentage in the latter half of 2008, 2009 and 2010 in Korea. Although the total number of AHA cases was increased, during weeks 32-52 of 2009, the mean weekly incidence percentage (AHA cases in a week/total in a year) was significantly lower than that during the same period in 2008 and 2010. |
 | Fig. 6Correlation between 2009 influenza A (H1N1) infection and acute hepatitis A (AHA) at the Soon Chun Hyang University Bucheon Hospital for weeks 32-52 of 2009. A significant negative correlation between 2009 H1N1 pandemic and AHA was detected at the SCH hospital during surveillance weeks 32-52 in 2009 (r=-0.597; p<0.05). |
References
1. 2009 H1N1 Flu ("Swine Flu") and You [Internet]. 2010. 02. 10. cited 2011 Jan 30. Atlanta: Centers for Disease Control and Prevention (CDC);Available from: http://www.cdc.gov/h1n1flu/qa.htm.
2. What is the pandemic (H1N1) 2009 virus? [Internet]. 2010. 02. 24. cited 2011 Jan 30. Genova: World Health Organization (WHO);Available from: http://www.who.int/csr/disease/swineflu/frequently_asked_questions/about_disease/en/index.html.
3. Influenza A (H1N1) - update 9 [Internet]. 2009. 05. 02. cited 2011 Jan 30. Genova: World Health Organization (WHO;Available from: http://www.who.int/csr/don/2009_05_02/en/index.html.
4. Feinstone SM, Kapikian AZ, Purceli RH. Hepatitis A: detection by immune electron microscopy of a viruslike antigen associated with acute illness. Science. 1973. 182:1026–1028.
5. Sohn YM, Rho HO, Park MS, et al. The changing epidemiology of hepatitis A in children and the consideration of active immunization in Korea. Yonsei Med J. 2000. 41:34–39.
6. Jeong SH. Current status and vaccine indication for hepatitis. A virus infection in Korea. Korean J Gastroenterol. 2008. 51:331–337.
7. Martin A, Lemon SM. Hepatitis A virus: from discovery to vaccines. Hepatology. 2006. 43:2 Suppl 1. S164–S172.
8. Hepatitis A [Internet]. 2008. 05. cited 2011 Jan 30. Genova: World Health Organization (WHO);Available from: http://www.who.int/mediacentre/factsheets/fs328/en/index.html.
9. Korea Centers for Disease Control and Prevention. Changing patterns of hepatitis A virus infection in Korea. Public Health Wkly Rep. 2008. 1:169–172.
10. Kiely PM, Lian KY, Napper G, Lakkis C. Influenza A (H1N1) and infection control guidelines for optometrists. Clin Exp Optom. 2009. 92:490–494.
11. Bell BP. Hepatitis A vaccine. Semin Pediatr Infect Dis. 2002. 13:165–173.
12. Innis BL, Snitbhan R, Kunasol P, et al. Protection against hepatitis A by an inactivated vaccine. JAMA. 1994. 271:1328–1334.
13. Hammitt LL, Bulkow L, Hennessy TW, et al. Persistence of antibody to hepatitis A virus 10 years after vaccination among children and adults. J Infect Dis. 2008. 198:1776–1782.
14. Dagan R, Leventhal A, Anis E, Slater P, Ashur Y, Shouval D. Incidence of hepatitis A in Israel following universal immunization of toddlers. JAMA. 2005. 294:202–210.
15. Belmaker I, Dukhan L, Yosef Y, Leventhal A, Dagan R. Elimination of hepatitis a infection outbreaks in day care and school settings in southern Israel after introduction of the national universal toddler hepatitis a immunization program. Pediatr Infect Dis J. 2007. 26:36–40.
16. Tong MJ, el-Farra NS, Grew MI. Clinical manifestations of hepatitis A: recent experience in a community teaching hospital. J Infect Dis. 1995. 171:Suppl 1. S15–S18.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download