Abstract
Background/Aims
Hemobilia is a rare cause of upper gastrointestinal bleeding. Endoscopic retrograde cholangiopancreaticography (ERCP) is considered to be an excellent diagnostic and treatment modality. Thirty-seven cases of hemobilia with different underlying pathologies were analyzed to illustrate clinical features and to evaluate the role of endoscopic management.
Methods
A total of 37 patients (26 men and 11 women; mean age, 66.2±15.3 years) who were confirmed to have hemobilia by ERCP in a single center from 2000 to 2010 were reviewed retrospectively. Patients with iatrogenic causes of hemobilia were excluded in this study.
Results
The causes of hemobilia were hepatocellular carcinoma in 14, bile duct and gallbladder malignancies in 12, common bile duct stones with cholangitis in 4, acute cholecystitis in 4, and pancreatic cancer in 2 patients. The clinical features of hemobilia were jaundice (89.2%), abdominal pain (78.4%), and melena (13.5%). The cholangiographic findings of hemobilia were amorphous filling defects in 15, tubular filling defects in 6, and cast-like filling defects in 6 patients. Endoscopic management included endoscopic nasobiliary drainage in 26 patients and endoscopic retrograde biliary drainage in 7 patients. Biliary obstruction caused by hemobilia was successfully treated with endoscopic biliary drainages in most cases.
Since the term 'hemobilia' was first used by Sandblom1 in 1948, hemobilia has been more frequently diagnosed owing to the various diagnostic tools and increasing knowledge of this condition. Hemobilia occurs if there is a communication or fistula between a biliary duct and a blood vessel. Hemobilia is a rare cause of upper gastrointestinal bleeding, and can arise from any biliary system, including intra- and extra-hepatic bile ducts, gallbladder, pancreas, or ampullary orifice. The typical clinical features of abdominal pain, jaundice, and gastrointestinal bleeding are known as the 'Quinke triad' of hemobilia.2 Moreover, hemobilia is frequently associated with biliary obstruction and subsequent acute cholangitis.
In the past, the most common cause of hemobila was accidental trauma, followed by iatrogenic injury, such as liver biopsy or percutaneous transhepatic cholangiography (PTC). Various conditions associated with hemobilia, such as cholecystitis, cholangitis, coagulopathies, vascular malformations, and hepatobiliary tumors, have been increasingly reported. A high index of suspicion is needed for the diagnosis of hemobilia. Unless there is a history of trauma or injury, hemobilia is not easy to recognize in the early stage.3-5 Of the available modalities, ERCP has played a significant role in the diagnosis and treatment of hemobilia. The purpose of this study was to analyze 37 cases of non-iatrogenic hemobilia, which were all confirmed by ERCP in our hospital, to identify the etiology and clinical manifestations of hemobilia, and to present the role for endoscopic management of hemobilia.
Thirty-seven patients with hemobilia diagnosed at Yeungnam University Hospital from January 2000 to December 2010 were analyzed retrospectively. Hembilia was confirmed with ERCP in all patients. Patients with iatrogenic causes of hemobilia developed after liver biopsy, PTC or biliary cannulation during ERCP were excluded in this study. The medical records and endoscopic and radiologic findings of all patients were reviewed. The clinical symptoms, blood chemistry, causes of hemobilia, endoscopic management, and cholangiographic findings were documented. The protocol was approved by the institutional review board of our hospital. ERCP was performed by experienced endoscopists using side-view endoscopes (TJF-240; Olympus Optical Corporation, Tokyo, Japan). The diagnosis of hemobilia was made when fresh blood or blood clots were evident via the ampullary orifice from the bile duct. After identifying hemobilia, we attempted to take an appropriate cholangiogram on cannulation by ERCP. Following the optimum cholangiogram, an endoscopic nasobiliary drainage (ENBD) or endoscopic retrograde biliary drainage (ERBD) was placed according to the cholangiographic findings and patient conditions. ENBD was performed if the biliary system was filled with blood clots or ongoing bleeding was suspected. ERBD was performed to prevent cholangitis due to retained blood clot or rebleeding when there was only minor bleeding and cessation of bleeding was expected. Antibiotics were given in all patients and packed cell transfusions were administered as needed.
The mean age was 66.2±15.3 years (range, 26-89 years). The male-to-female ratio was 2.4 : 1. Liver enzyme studies showed the following mean values: total bilirubin, 10.5 mg/dL; aspartate aminotransferase, 353 IU/L; alanine aminotransferase, 243 IU/L; alkaline phosphatase, 834 IU/L; and gamma glutamyl transpeptidase, 385 IU/L. A complete blood count revealed a mean hemoglobin level of 10.6 g/dL (range, 7.3-15.8 g/dL). Eight patients (21.6%) required packed red cell transfusion (Table 1). The most frequent symptoms and signs were jaundice in 33 patients (89.2%), followed by abdominal pain in 29 patients (78.4%), and melena in 5 patients (13.5%). All of the patients that presented with melena had abdominal pain and jaundice. Hematemesis was not observed in our patients.
Of the 37 cases, there were 28 cases of malignancy and 8 cases of inflammation. Among the hemobilia associated with malignancy, 14 cases were due to hepatocellular carcinoma, 10 cases due to cholangiocarcinoma, 2 cases due to gallbladder cancer, and remaining 2 cases due to pancreatic cancer. Among the 8 cases of inflammation, 4 cases were caused by common bile duct (CBD) stones with cholangitis, 3 cases were caused by acute calculous cholecystitis, and 1 case was caused by acalculous hemorrhagic cholecystitis. The cause of the remaining 1 case was unknown (Table 2).
Endoscopic retrograde cholangiograms (ERCs) were performed in 34 cases. The cholangiograms revealed various forms of filling defects in the dilated bile ducts. The most common cholangiographic features were amorphous filling defects in 15 cases, followed by tubular filling defects in 6 cases, and cast-like filling defects in 6 cases (Fig. 1A-C). There were 5 cases of CBD dilatation with no demonstrable filling defects and 2 cases of gallbladder filling defects without CBD involvement. Of tumor-related hemobilia, 6 of 10 patients with cholangiocarcinoma showed amorphous filling defects, and 11 of 14 patients with hepatocellular carcinoma showed tubular or cast-like filling defects. Of the 5 patients with CBD dilation with no demonstrable filling defects, 2 patients had underlying liver cirrhosis. The remaining 3 patients showed that large amounts of blood admixed with the bile was gushed out immediately after ERBD, representing massive hemobilia (Fig. 1D, E). Two patients with gallbladder cancer showed amorphous filling defects within distended gallbladders (Fig. 1F).
Following the cholangiogram, blood clots were removed with balloon catheters or stone retrieval baskets as much as possible. ENBD or ERBD was placed in 26 (70.3%) and 7 patients (18.9%), respectively. Only endoscopic sphincterotomies were applied in two patients with minor hemobilia. In two cases of hemobilia, ERCP was failed and percutaneous transhepatic biliary drainage was subsequently performed. A follow-up cholangiogram was performed in patients who underwent ENBD. At the time of the follow-up cholangiogram, there was no evidence of ongoing bleeding and most blood clots disappeared in all cases. One case of hepatocellular carcinoma had two episodes of hemobilia in a series; in this case, an ERBD was placed initially, then it was changed into ENBD during the follow-up ERCP due to ongoing bleeding.
After stabilization of hemobilia and improvement of cholangitis, patients were treated according to the underlying diseases causing hemobilia.
Six patients underwent cholecystectomies (2 open cholecystectomies due to gallbladder cancer, and 4 laparoscopic cholecystectomies due to acute cholecystitis). A pylorus-preserving pancreatoduodenectomy was performed in one patient with pancreatic head cancer. Among the 14 cases of hepatocellular carcinoma, 2 were treated with lobectomies, 2 were treated with transarterial chemoembolization (TAE), 2 were treated with intra-arterial chemotherapy, and 1 was treated with TAE combined with a lobectomy (Table 3). Those patients who were unfit for surgery were treated conservatively.
There were 4 (10.5%) in-hospital mortalities related to hemobilia in the present study. Two patients died from sepsis due to the aggravation of suppurative cholangitis. One patient died from aggravating hepatic encephalopathy as a complication of liver cirrhosis. The remaining one patient died from hepatic failure, sepsis and multi-organ failure. However, no mortalities were directly related to the bleeding.
Hemobilia, although uncommon, is challenging to recognize because the diagnosis and management is quite different from that of other causes of gastrointestinal bleeding. Abdominal pain, melena or hematemesis, and obstructive jaundice are widely indicated as a typical triad of symptoms, presenting in 22-37.9% of hemobilia patients.6,7 Hemobilia usually involves minor bleeding and stops spontaneously in most cases. In the case of rapid bleeding, the blood flows rapidly into the duodenum, presenting as melena or hematemesis. In contrast, slow bleeding has the tendency to form clots and cause biliary obstruction because the blood does not mix with bile and forms separate layers due to the difference in gravity and surface tension.8 The triad of symptoms occurred in only 13.5% of our cases. In the present study, abdominal pain and jaundice, indicating symptoms related to bile duct obstruction, were predominant symptoms; however, there were no cases presenting with hematemesis. The absence of hematemesis might be explained as follows: first, there was no massive bleeding in our cases; and second, the pyloric sphincter might prevent blood from the duodenum regurgitating into the stomach unless there was profuse bleeding.
In the past, the causes of hemobilia were accidental trauma in 38.6% of patients, followed by gallbladder stones in 14.9%, inflammation in 13%, vascular disorder in 10.7%, and tumors in 6.2% of the patients.9 There has been a shift in the etiology of hemobilia over time. Currently, the main etiology of hemobilia is iatrogenic, of which needle biopsy or percutaneous intervention account for the main portion of cases of hemobilia due to increasing demand.5-7,10 Other causes such as cholecystitis, cholangitis, parasitic infestation, and hepatobiliary tumors were infrequently associated with hemobilia. Hepatobiliary malignancies, including hepatocellular carcinoma, were predominant causes of non-iatrogenic hemobilia in our study. Although we excluded hemobilia associated with percutaneous interventions of the bile duct or liver biopsy in this study, we experienced many cases of hemobilia occurring after PTC or liver biopsy in our hospital during the same study period. Most of hemobila caused by PTC were usually controlled spontaneously and significant bleeding necessitating embolization was very rare (data not shown). Vessel injuries associated with liver biopsy or percutaneous intervention may have mostly been avoided with the help of ultrasonographic guidance.
Cholelithiasis is frequently associated with microscopic bleeding of the gallbladder and gallstone-related hemobilia accounts for 9% of reported cases of hemobilia.7 Of the cases, 18.9% were related with cholangitis associated with CBD stones or cholecystitis in the present study. The stones in the gallbladder or CBD make direct mucosal damage and erode the vessel of the cystic artery, which usually leads to minor bleeding.11 Moreover, acalculous cholecytitis may lead to hemobilia as well. It is postulated that the high pressure associated with acute cholecystits might cause erosion of gallbladder mucosa, subsequent ulceration of the vessel wall, and then bleeding into the bile duct.12,13 Neoplasm of the hepatobiliary tract is another important cause of hemobilia, with a reported incidence of 8.7%.7 There have been increasing reports of malignancy-related hemobilia, including hepatocellular carcinoma, cholangiocarcinoma, pancreatic cancer, gallbladder cancer, and hepatic metastasis.14,15 Hepatobiliary tumors, especially in the advanced stage, tend to cause hemobilia by direct invasion of surrounding vessels, not by the tumor itself.16,17 In our study, the incidence of tumor-related hemobilia was 75.6% of non-iatrogenic hemobilia, and 50% of the patients with the tumor-related hemobilia were associated with hepatocelluar carcinoma. The currently available diagnostic modalities include esophagogastroduodenoscopy, side-view endoscopy, abdominal CT, ultrasonography, and angiography.18 Although CT angiography is very helpful to detect the bleeding point and mass lesions, it does not indicate the amount and rate of bleeding. An endoscopy has many advantages in that endoscopy can demonstrate fresh blood around the ampulla of Vater and exclude the other bleeding sites in the gastrointestinal tract. ERCP has played important roles in the diagnosis of hemobilia.19 First, the color, rate, and amount of bleeding can be assessed by direct visualization. Second, cholangiogragm of the biliary tree could reveal the causes of hemobilia, type and site of filling defects, and degree of bile duct dilatation.
ERC shows characteristic filling defects in the biliary tree or gallbladder in most cases of hemobilia. The morphology of these defects is diverse. In most cases, the characteristic findings by ERCP are amorphous, tubular, or cast-like in the bile ducts as blood clots do not mix with bile easily and remain separate from the bile. However, in some cases with massive hemobilia or underlying liver cirrhosis in our study, CBD dilatation was the only cholangiographic feature of hemobilia and there were no filling defects suggesting blood clots in the biliary tree. An underlying coagulopathy or massive bleeding may explain the impaired formation of blood clots and admixture of bile and large amounts of blood can cause bile duct dilatation in these patients. Of malignancy-related hemobilia, amorphous filling defects were shown in 60% of the patients with cholangiocarcinoma; however, tubular or cast-like filling defects were present in 78.5% of the patients with hepatocellular carcinoma. In the current study, several cases of hemobilia showed heterogeneous filling defects in the gallbladder, suggesting bleeding that originated from the gallbladder. However, blood clots in the gallbladder did not always indicate bleeding derived from the gallbladder because significant blood from the bile duct can enter the gallbladder and form amorphous blood clots.
The main goal for the treatment of hemobilia is directed at hemostasis and restoring the bile flow. When the bile flow is interrupted, the clot does not dissolve and remains in the bile duct due to the disturbance of the fibrinolytic action of the bile.6,20-22 Biliary decompression is required first, unless patients are hemodynamically unstable due to ongoing massive bleeding. Although minor hemobilia can be managed conservatively with correction of coagulopathy and fluid hydration, hemobilia usually requires endoscopic therapy, radiologic intervention, or surgery.
Currently, ERCP has played a great role in the management of hemobilia. Removal of blood clots from the bile duct is initially required to maintain the bile flow. After sphincterotomy, the extraction of blood clots from the biliary tree can relieve the abdominal pain and jaundice.23,24 Clot formation in the bile duct may result in acute cholecystitis, cholangitis, and acute pancreatitis secondary to biliary obstruction.25 These drainage catheters can prevent cholangitis by decompression of the bile duct and lessen the rate of bleeding as well. Cholangitis can be fatal if not treated aggressively with biliary decompression and broad-spectrum antibiotics. After placing an endoscopic prosthesis, the abdominal pain was notably relieved and liver enzyme including total bilirubin was subsequently improved. The majority of patients did not require immediate surgery to control hemorrhage in our experience, indicating that biliary decompression itself may be sufficient to stop the bleeding. In particular, ENBD is preferred to ERBD in the clinical setting. ENBD permits monitoring the drainage of blood, irrigation of the bile duct, and confirmation of clearance of the bile duct by the follow-up cholangiogram, which can help to avoid repeat endoscopies.26 ERCP can also be considered as a primary choice in patients with significant hemobilia because it may function as a bridge to further therapy.
With respect to the treatment of tumor-related hemobilia, other therapeutic modalities, including TAE, chemotherapy, or surgery, are options. In recent years, TAE is preferred to surgery, because TAE is less invasive and more effective, with a success rate of 80-100%.6,10,27 Nevertheless, surgical treatment should be considered if embolization is unsuccessful or there are inflammatory diseases of the biliary system. Cholecystectomy or CBD stone removal should be considered if hemobilia is associated with calculous cholecystitis or hemorrhagic cholecystitis.25 The prognosis of hemobilia is largely dependent on the underlying causes. The prognosis of hemobilia associated with gallstones is good after cholecystectomy, however, that of hemobilia associated with malignancy is poor.28 Recently, the mortality rate related to hemobilia has dropped from 12% to 5%.6,7 Although our in-hospital mortality rate was 10.5%, the causes of death were related to the aggravation of cholangitis or underlying diseases, but not to the severity of blood loss.
In conclusion, hemobilia, still an uncommon clinical manifestation, should be considered in the differential diagnosis of gastrointestinal bleeding and biliary obstruction. The most common non-iatrogenic causes of hemobilia were hepatobiliary malignancies and usually presented by biliary obstruction due to blood clots. This study suggests, whatever the cause, ERCP is a feasible and effective method in patients with hemobilia because ERCP cannot only confirm the hemobilia, but also manage the bleeding and bile duct obstruction. Therefore, endoscopic biliary drainage is recommended as the initial management of hemobilia to control biliary obstruction.
Figures and Tables
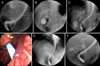 | Fig. 1Endoscopic retrograde cholangiographic findings of hemobilia. (A) In a 59-year-old male with hepatocellular carcinoma, cholangiogram showed a large, cast-like filling defect in the dilated common bile duct. (B) In a 68-year-old male with hepatocellular carcinoma, cholangiogram showed a longitudinal, tubular filling defect in the common bile duct and the right main intrahepatic duct. (C) In a 78-year-old male with common bile duct cancer, cholangiogram showed ill-defined, amorphous filling defects in the proximal common bile duct and bifurcation of the bile duct. (D, E) In a 79-year-old male with acute suppurative cholangitis, ERCP demonstrated that large amounts of blood admixed with pustulous bile was gushing out after endoscopic retrograde biliary drainage, but, cholangiogram showed no demonstrable filling defect in the dilated common bile duct. (F) In a 75-year-old male with gallbladder cancer, cholangiogram showed amorphous filling defects within the distended gallbladder with no demonstrable filling defect in the common bile duct. |
References
1. Sandblom P. Hemorrhage into the biliary tract following trauma; traumatic hemobilia. Surgery. 1948. 24:571–586.
2. Quincke H. Ein fall von aneurysma der leberarterie. Berl Klein Wochenschr. 1871. 30:349–352.
3. Alis H, Bozkurt MA, Oner OZ, et al. Case report: acute pancreatitis caused by postcholecystectomic hemobilia. BMC Gastroenterol. 2010. 10:75.
4. Park SS, Kim BU, Han HS, et al. Hemobilia from ruptured hepatic artery aneurysm in polyarteritis nodosa. Korean J Intern Med. 2006. 21:79–82.
5. Hendriks MP, Wanten GJ, Drenth JP. Management of hemobilia and pancreatitis after liver biopsy: a key role for endoscopic retrograde cholangiopancreaticography. Liver Transpl. 2009. 15:1653–1654.
6. Green MH, Duell RM, Johnson CD, Jamieson NV. Haemobilia. Br J Surg. 2001. 88:773–786.
7. Yoshida J, Donahue PE, Nyhus LM. Hemobilia: review of recent experience with a worldwide problem. Am J Gastroenterol. 1987. 82:448–453.
8. Sandblom P, Mirkovitch V. Minor hemobilia. Clinical significance and pathophysiological background. Ann Surg. 1979. 190:254–264.
9. Sandblom P. Hemobilia (Biliary Tract Hemorrhage): History, Diagnosis, and Treatment. 1972. Springfield: Charles C Thomas.
10. Chin MW, Enns R. Hemobilia. Curr Gastroenterol Rep. 2010. 12:121–129.
11. Bloechle C, Izbicki JR, Rashed MY, et al. Hemobilia: presentation, diagnosis, and management. Am J Gastroenterol. 1994. 89:1537–1540.
12. Willemsen PJ, Vanderveken ML, De Caluwe DO, Tielliu IF. Hemobilia: a rare complication of cholecystitis and cholecystolithiasis. Case report. Acta Chir Belg. 1996. 96:93–94.
13. Brady E, Welch JP. Acute hemorrhagic cholecystitis causing hemobilia and colonic necrosis. Dis Colon Rectum. 1985. 28:185–187.
14. Merrell SW, Schneider PD. Hemobilia-evolution of current diagnosis and treatment. West J Med. 1991. 155:621–625.
15. Hasan S, Quintana J, Siddiqui Y, Maldonado V. Carcinoma of the pancreas presenting with hemobilia. Gastrointest Endosc. 1986. 32:305–306.
16. Qin LX, Tang ZY. Hepatocellular carcinoma with obstructive jaundice: diagnosis, treatment and prognosis. World J Gastroenterol. 2003. 9:385–391.
17. Kojiro M, Kawabata K, Kawano Y, Shirai F, Takemoto N, Nakashima T. Hepatocellular carcinoma presenting as intrabile duct tumor growth: a clinicopathologic study of 24 cases. Cancer. 1982. 49:2144–2147.
18. Sax SL, Athey PA, Lamki N, Cadavid GA. Sonographic findings in traumatic hemobilia: report of two cases and review of the literature. J Clin Ultrasound. 1988. 16:29–34.
19. Kroser J, Rothstein RD, Kochman ML. Endoscopic management of obstructive jaundice caused by hemobilia. Gastrointest Endosc. 1996. 44:618–619.
20. Sandblom P, Mirkovitch V, Saegesser F. Formation and fate of fibrin clots in the biliary tract: a clinical and experimental study. Ann Surg. 1977. 185:356–366.
21. Sandblom P, Saegesser F, Mirkovitch V. Hepatic hemobilia: hemorrhage from the intrahepatic biliary tract, a review. World J Surg. 1984. 8:41–50.
22. Luzuy F, Reinberg O, Kauszlaric D, et al. Biliary calculi caused by hemobilia. Surgery. 1987. 102:886–889.
23. Worobetz LJ, Passi RB, Sullivan SN. Hemobilia after percutaneous liver biopsy: role of endoscopic retrograde cholangiopancreatography and sphincterotomy. Am J Gastroenterol. 1983. 78:182–184.
24. Jensen AR, Matzen P. Hemobilia with jaundice: treatment by endoscopic papillotomy. Am J Gastroenterol. 1982. 77:162–163.
25. Lee SL, Caruso DM. Acute cholecystitis secondary to hemobilia. J Laparoendosc Adv Surg Tech A. 1999. 9:347–349.
26. Baker AR, Corlett SK, Cookson JB, Carr-Locke DL. Hemobilia treated by nasobiliary catheterization. Am J Gastroenterol. 1987. 82:783–785.
27. Srivastava DN, Sharma S, Pal S, et al. Transcatheter arterial embolization in the management of hemobilia. Abdom Imaging. 2006. 31:439–448.
28. Goodnight JE Jr, Blaisdell FW. Hemobilia. Surg Clin North Am. 1981. 61:973–979.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download