Abstract
Background/Aims
Entecavie (ETV) has a potent antiviral effect and low rates of resistance in hepatitis B virus (HBV) and is the first-line monotherapy in patients with HBV-related decompensated cirrhosis. We evaluated the efficacy of 12 months treatment with ETV and tried to determine predictive factors of response.
Methods
Forty-five consecutive decompensated cirrhotic patients who received ETV (0.5 mg/day) for more than six months were included. All patients were positive for HBV DNA, and the Child-Turcotte-Pugh (CTP) scores were over 8 point. Seventeen patients were HBeAg-positive. CTP score, model for end-stage liver disease (MELD) score, serum markers of liver function and HBV DNA were assessed every 3 months.
Results
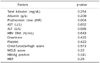
ETV treatment for 12 months resulted in improvement of CTP and MELD scores. Pre-treatment mean CTP and MELD score were decreased from 10.1 (±2.0) and 13.48 (±4.05) to 7.24 (±2.0) and 9.68 (±4.85) at 12 months, respectively. The 1-year cumulative rates of HBV DNA negativity and HBeAg loss were 88.9% and 52.9%, respectively, by intention-to-treat analysis. Thirty-two (71.1%) showed improvement in CTP score. Eleven patients did not show change, and 2 patients got worse. The AST/ALT, albumin, bilrubin, prothrombin time were significantly normalized within six months. The good responder group had high level of prothrombin time than the poor responder group (p=0.004).
Figures and Tables
 | Fig. 2Changes of Child-Turcotte-Pugh (CTP) score and model for end stage liver disease (MELD) score during entecavir therapy. |
Table 3
Comparison of Virologic and Biochemical Response to Entecavir between the Responder and Non-Responder Groups

Values are presented as mean±SD. The results were used by an item deletion method about missing values in each follow-up data of every interest factors.
R, responder group, defined as a decrease in CTP score of ≥2; NR, non-responder group, defined as a decrease in CTP score of ≤1 or an increase; CTP, Child-Turcotte-Pugh; MELD, model for end stage liver disease.
Notes
References
1. Schiff ER. Prevention of mortality from hepatitis B and hepatitis C. Lancet. 2006. 368:896–897.
2. Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004. 350:1118–1129.
3. Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology. 2007. 45:1056–1075.
4. Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006. 295:65–73.
5. Fattovich G, Pantalena M, Zagni I, Realdi G, Schalm SW, Christensen E. Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. Am J Gastroenterol. 2002. 97:2886–2895.
6. Kim JI, Na JS, Bang CS, et al. Study of long term follow- up of interferon therapy in chronic viral hepatitis - in 222 cases during 127 weeks-. Korean J Hepatol. 1997. 3:241–251.
7. Yeon JE, Seo YS, Kim YH, et al. Long-term Follow-up of Patients Treatedwith Interferon Alfa for Chronic Hepatitis B. Korean J Hepatol. 1999. 5:12–21.
8. Lee WM. Hepatitis B virus infection. N Engl J Med. 1997. 337:1733–1745.
9. Perrillo R, Tamburro C, Regenstein F, et al. Low-dose, titratable interferon alfa in decompensated liver disease caused by chronic infection with hepatitis B virus. Gastroenterology. 1995. 109:908–916.
10. Hoofnagle JH, Di Bisceglie AM, Waggoner JG, Park Y. Interferon alfa for patients with clinically apparent cirrhosis due to chronic hepatitis B. Gastroenterology. 1993. 104:1116–1121.
11. Dienstag JL, Schiff ER, Wright TL, et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999. 341:1256–1263.
12. Lai CL, Chien RN, Leung NW, et al. Asia Hepatitis Lamivudine Study Group. A one-year trial of lamivudine for chronic hepatitis B. N Engl J Med. 1998. 339:61–68.
13. Shaw T, Bartholomeusz A, Locarnini S. HBV drug resistance: mechanisms, detection and interpretation. J Hepatol. 2006. 44:593–606.
14. Keeffe EB, Dieterich DT, Han SH, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: an update. Clin Gastroenterol Hepatol. 2006. 4:936–962.
15. Liaw YF, Leung N, Guan R, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2005 update. Liver Int. 2005. 25:472–489.
16. Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004. 351:1521–1531.
17. Chang TT, Gish RG, de Man R, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006. 354:1001–1010.
18. Lai CL, Shouval D, Lok AS, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006. 354:1011–1020.
19. Shim JH, Lee HC, Kim KM, et al. Efficacy of entecavir in treatment-naive patients with hepatitis B virus-related decompensated cirrhosis. J Hepatol. 2010. 52:176–182.
20. Saldanha J, Gerlich W, Lelie N, Dawson P, Heermann K, Heath A. An international collaborative study to establish a World Health Organization international standard for hepatitis B virus DNA nucleic acid amplification techniques. Vox Sang. 2001. 80:63–71.
21. Kitay-Cohen Y, Ben-Ari Z, Tur-Kaspa R, Fainguelernt H, Lishner M. Extension of transplantation free time by lamivudine in patients with hepatitis B-induced decompensated cirrhosis. Transplantation. 2000. 69:2382–2383.
22. Keeffe EB. End-stage liver disease and liver transplantation: role of lamivudine therapy in patients with chronic hepatitis B. J Med Virol. 2000. 61:403–408.
23. Yao FY, Bass NM. Lamivudine treatment in patients with severely decompensated cirrhosis due to replicating hepatitis B infection. J Hepatol. 2000. 33:301–307.
24. Villeneuve JP, Condreay LD, Willems B, et al. Lamivudine treatment for decompensated cirrhosis resulting from chronic hepatitis B. Hepatology. 2000. 31:207–210.
25. Kapoor D, Guptan RC, Wakil SM, et al. Beneficial effects of lamivudine in hepatitis B virus-related decompensated cirrhosis. J Hepatol. 2000. 33:308–312.
26. Yao FY, Terrault NA, Freise C, Maslow L, Bass NM. Lamivudine treatment is beneficial in patients with severely decompensated cirrhosis and actively replicating hepatitis B infection awaiting liver transplantation: a comparative study using a matched, untreated cohort. Hepatology. 2001. 34:411–416.
27. Leung N. Recent data on treatment of chronic hepatitis B with nucleos(t)ide analogues. Hepatol Int. 2008. 2:163–178.
28. Liaw YF. Results of lamivudine trials in Asia. J Hepatol. 2003. 39:Suppl 1. S111–S115.
29. Tenney DJ, Rose RE, Baldick CJ, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapy. Hepatology. 2009. 49:1503–1514.
30. Jung S, Suh DJ, Park HJ, et al. Therapeutic efficacy of lamivudine in patients with hepatitis B virus-related decompensated cirrhosis in Korea. Korean J Hepatol. 2002. 8:418–427.
31. Liaw YF, Sheen IS, Lee CM, et al. Tenofovir disoproxil fumarate (TDF), emtricitabine/TDF, and entecavir in patients with decompensated chronic hepatitis B liver disease. Hepatology. 2011. 53:62–72.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download