Abstract
Bevacizumab (Avastin®) is a monoclonal antibody against the vascular endothelial growth factor (VEGF) receptor that increases the overall survival rate when added to standard chemotherapy regimens in patients with metastatic colorectal cancer. The known toxicities of bevacizumab are hypertension, proteinuria, wound healing complications, arterial thrombosis, bleeding, and gastrointestinal complications. Especially ischemic colitis can rapidly develop into bowel perforation, so an emergency operation often is needed. Recently, a 65-year-old male patient developed ischemic pancolitis after FOLFOX (85 mg/m2 Oxaliplatin, d1; 200 mg/m2 Leucovorin, d1; 400 mg/m2 5-FU iv bolus, d1-2; and 600 mg/m2 5-FU, d1-2, every two wk) and Bevacizumab combination chemotherapy was administered. However, he recovered after early conservative care without surgery. We report this case with a review of literature.
References
1. Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003; 21:60–65.

2. Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007; 25:1539–1544.

3. Gordon MS, Cunningham D. Managing patients treated with bevacizumab combination therapy. Oncology. 2005; 69(Suppl 3):25–33.

4. Scappaticci FA, Skillings JR, Holden SN, et al. Arterial throm-boembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007; 99:1232–1239.

5. Lordick F, Geinitz H, Theisen J, Sendler A, Sarbia M. Increased risk of ischemic bowel complications during treatment with bevacizumab after pelvic irradiation: report of three cases. Int J Radiat Oncol Biol Phys. 2006; 64:1295–1298.

6. Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol. 2009; 10:559–568.

7. Paran H, Edelstein E, Klein B, Gutman M. Extensive colonic ischemia following treatment with bevacizumab, fluouracil and CPT-11 in a young patient with advanced adenocarcinoma of the rectum. Isr Med Assoc J. 2007; 9:488–489.
8. Heinzerling JH, Huerta S. Bowel perforation from bevacizumab for the treatment of metastatic colon cancer: incidence, etiology, and management. Curr Surg. 2006; 63:334–337.

9. Kube R, Meyer F, Bien N, et al. Surgical management of bevacizumab-associated peritonitis due to perforation. Zentralbl Chir. 2009; 134:462–467.
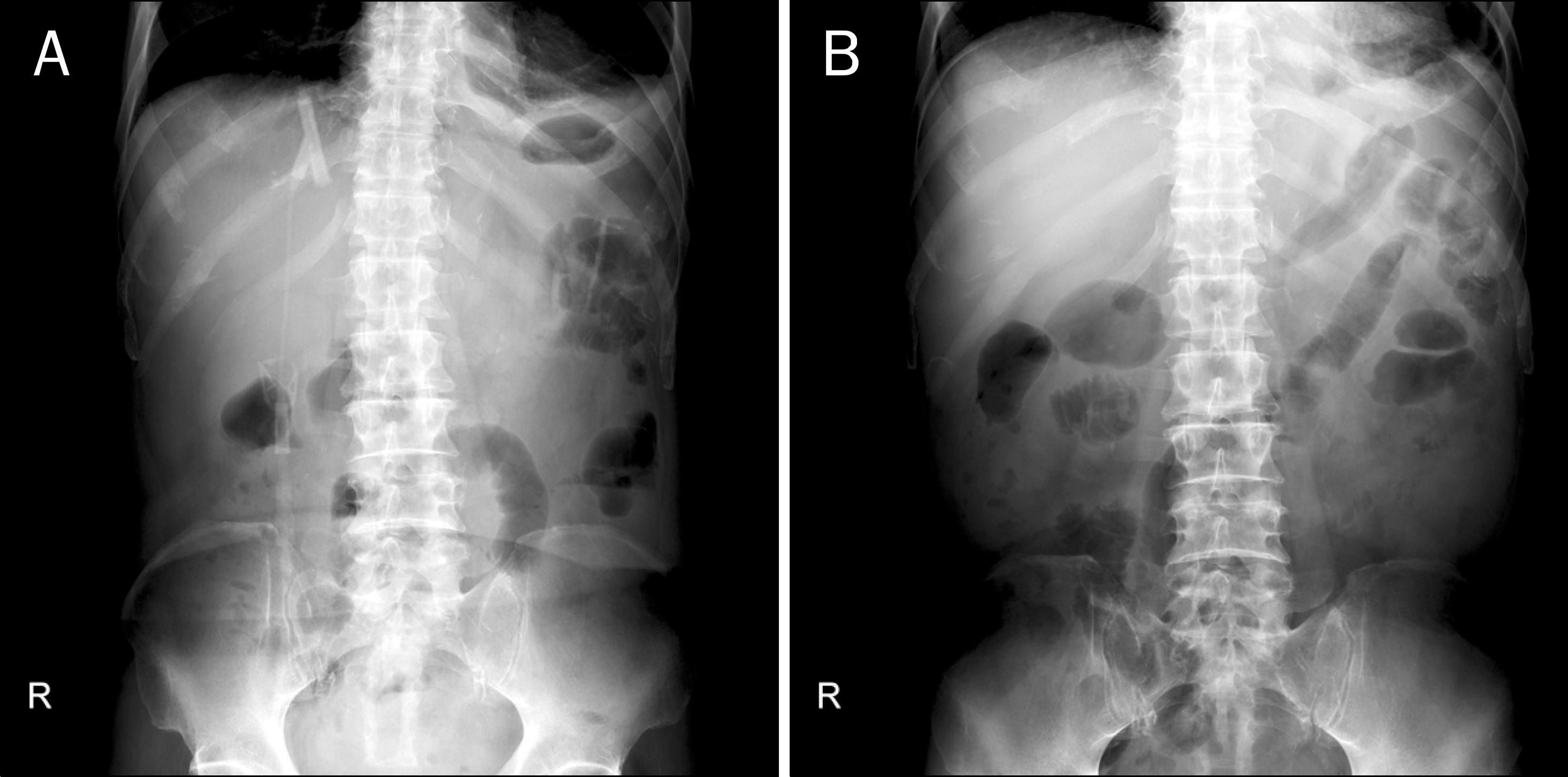
Fig. 1.
Abdominal simple x-ray. It showed the distension of the transver-se colon, and small and large bowel ileus.

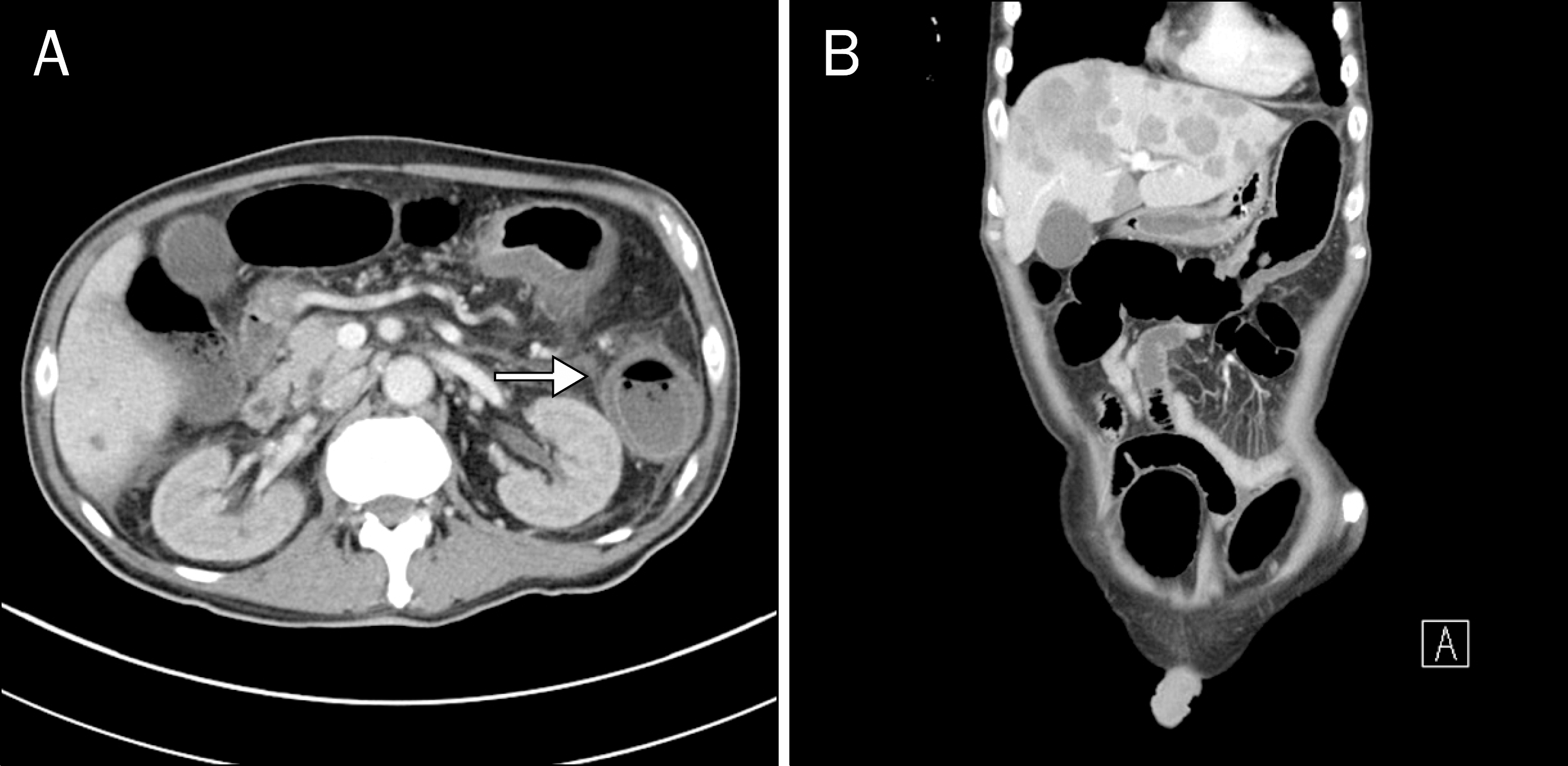
Fig. 2.
Abdominal CT scan. It showed the concentric layers of low and high attenuation (double-halo sign) (white arrow) and mucosal and serosal enhancement with edematous and non-enhanced thickening of submucosal layer from the ascending colon to sigmoid colon without evidence of perforation, suggesting colonic ischemia.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download