Abstract
Background/Aims
We retrospectively analyzed comparative toxicities and efficacies of chemotherapy regimens in advanced gastric cancer (AGC) patients who achieved complete response (CR) after chemotherapy.
Methods
We reviewed the medical records of 1,203 patients, who were pathologically diagnosed as AGC in a single center between January 2001 and October 2007. On the basis of the Response Evaluation Criteria in Solid Tumors, CR was evaluated with abdominal computed tomography. Toxicities were evaluated using the National Cancer Institute's common toxicity criteria before each chemotherapy cycle.
Results
Among the 1,203 AGC patients enrolled in this study, 568 received chemotherapy and 635 received best supportive care. The major chemotherapy regimens were 5-fluorouracil, leucovorin and oxaliplatin (FOLFOX), docetaxel, cisplatin and 5-fluo-rouracil (DCF) and 5-fluorouracil, leucovorin and irinotecan (FOLFIRI). Among the 568 patients, 51 (9.0%) achieved CR (49 [8.6%] with FOLFOX [n=12], DCF [n=26], or FOLFIRI [n=11] and 2 [0.3%] with etoposide, leucovorin and 5-fluorouracil). For patients administered FOLFOX, DCF, and FOLFIRI, the median time to disease progression was 4 months (range, 1.8–59.5), 15 months (range, 2.9–31.2) and 10 months (range, 2.0–39.5), and the median survival times were 48 months (range, 5.9–74.0), 37 months (range, 14.0–86.0), and 30 months (range, 6.0–50.0), respectively. Grades 3–4 mucositis occurred mostly in patients administered DCF (n=8, 30.8%). Grades 3–4 leucopenia were observed in 1 (8.3%), 11 (42.3%), and 4 (36.4%) patients administered FOLFOX, DCF and FOLFIRI, respectively. No statistically significant differences were observed in the 3 regimens.
Go to : 
References
1. Souglakos J, Syrigos K, Potamianou A, et al. Combination of irinotecan (CPT-11) plus oxaliplatin (L-OHP) as first-line treatment in locally advanced or metastatic gastric cancer: a multicentre phase II trial. Ann Oncol. 2004; 15:1204–1209.

2. Coleman MP, Gatta G, Verdecchia A, et al. EUROCARE Working Group. EUROCARE-3 summary: cancer survival in Europe at the end of the 20th century. Ann Oncol. 2003; 14(Suppl 5):v128–149.

3. Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000; 50:7–33.

4. Wöhrer SS, Raderer M, Hejna M. Palliative chemotherapy for advanced gastric cancer. Ann Oncol. 2004; 15:1585–1595.

5. Glimelius B, Ekström K, Hoffman K, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997; 8:163–168.

6. Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer. 1993; 72:37–41.

7. Pyrhönen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer. 1995; 71:587–591.

8. Kim NK, Park YS, Heo DS, et al. A phase III randomized study of 5-fluorouracil and cisplatin versus 5-fluorouracil, doxorubicin, and mitomycin C versus 5-fluorouracil alone in the treatment of advanced gastric cancer. Cancer. 1993; 71:3813–3818.

9. Vanhoefer U, Rougier P, Wilke H, et al. Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: A trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol. 2000; 18:2648–2657.

10. Kulig J, Kolodziejczyk P, Sierzega M, et al. Adjuvant chemotherapy with etoposide, adriamycin and cisplatin compared with surgery alone in the treatment of gastric cancer: a phase III randomized, multicenter, clinical trial. Oncology. 2010; 78:54–61.

11. Sparano JA, Schwartz EL, Salva KM, Pizzillo MF, Wadler S, Wiernik PH. Phase II trial of etoposide, doxorubicin (Adriamycin), and cisplatin (EAP regimen) in advanced gastric cancer. Am J Clin Oncol. 1990; 13:374–378.

12. Petrioli R, Sabatino M, Roviello F, et al. Folinic acid, 5-fluorouracil and etoposide after curative resection for gastric cancer. Hepatogastroenterology. 2005; 52:1626–1630.
13. Li J, Lu M, Shen L, Zhang XD, Li Y. Oxaliplatin-based regimen for the treatment of advanced or metastatic gastric/esophagogastric junction cancer. Zhonghua Zhong Liu Za Zhi. 2009; 31:933–936.
14. Kang SH, Kim JI, Moon HS, et al. Oxaliplatin and leucovorin plus fluorouracil versus irinotecan and leucovorin plus fluorouracil combination chemotherapy as a first-line treatment in patients with metastatic or recurred gastric adenocarcinoma. Korean J Gastroenterol. 2010; 55:26–32.

15. Louvet C, André T, Tigaud JM, et al. Phase II study of oxaliplatin, fluorouracil, and folinic acid in locally advanced or metastatic gastric cancer patients. J Clin Oncol. 2002; 20:4543–4548.

16. Al-Batran SE, Atmaca A, Hegewisch-Becker S, et al. Phase II trial of biweekly infusional fluorouracil, folinic acid, and oxaliplatin in patients with advanced gastric cancer. J Clin Oncol. 2004; 22:658–663.

17. De Vita F, Orditura M, Matano E, et al. A phase II study of biweekly oxaliplatin plus infusional 5-fluorouracil and folinic acid (FOLFOX-4) as first-line treatment of advanced gastric cancer patients. Br J Cancer. 2005; 92:1644–1649.

18. Assersohn L, Brown G, Cunningham D, et al. Phase II study of irinotecan and 5-fluorouracil/leucovorin in patients with primary refractory or relapsed advanced oesophageal and gastric carcinoma. Ann Oncol. 2004; 15:64–69.

19. Cervantes A, Roselló S, Roda D, Rodríguez-Braun E. The treatment of advanced gastric cancer: current strategies and future perspectives. Ann Oncol. 2008; 19(Suppl 5):v103–v107.

20. Zhao JG, Qiu F, Xiong JP, et al. A phase II study of modified FOLFOX as first-line chemotherapy in elderly patients with advanced gastric cancer. Anticancer Drugs. 2009; 20:281–286.

21. Kim YS, Hong J, Sym SJ, et al. Oxaliplatin, 5-fluorouracil and leucovorin (FOLFOX-4) combination chemotherapy as a salvage treatment in advanced gastric cancer. Cancer Res Treat. 2010; 42:24–29.

22. Roth AD, Maibach R, Martinelli G, et al. Docetaxel (Taxotere)- cisplatin (TC): an effective drug combination in gastric carcinoma. Swiss Group for Clinical Cancer Research (SAKK), and the European Institute of Oncology (EIO). Ann Oncol. 2000; 11:301–306.
23. Ridwelski K, Gebauer T, Fahlke J, et al. Combination chemotherapy with docetaxel and cisplatin for locally advanced and metastatic gastric cancer. Ann Oncol. 2001; 12:47–51.

24. Kang SH, Kim JI, Goh PG, et al. Docetaxel-Cisplatin-5-FU combination chemotherapy as a first-line treatment in patients with metastatic or recurred gastric cancer. Korean J Gastroenterol. 2007; 50:157–163.
25. Kim BG, Oh SY, Kwon HC, et al. A phase II study of irinotecan with biweekly, low dose leucovorin and bolus and continuous infusion 5-fluorouracil (modified FOLFIRI) as first line therapy for patients with recurrent or metastatic gastric cancer. Am J Clin Oncol. 2010; 33:246–250.

Go to : 
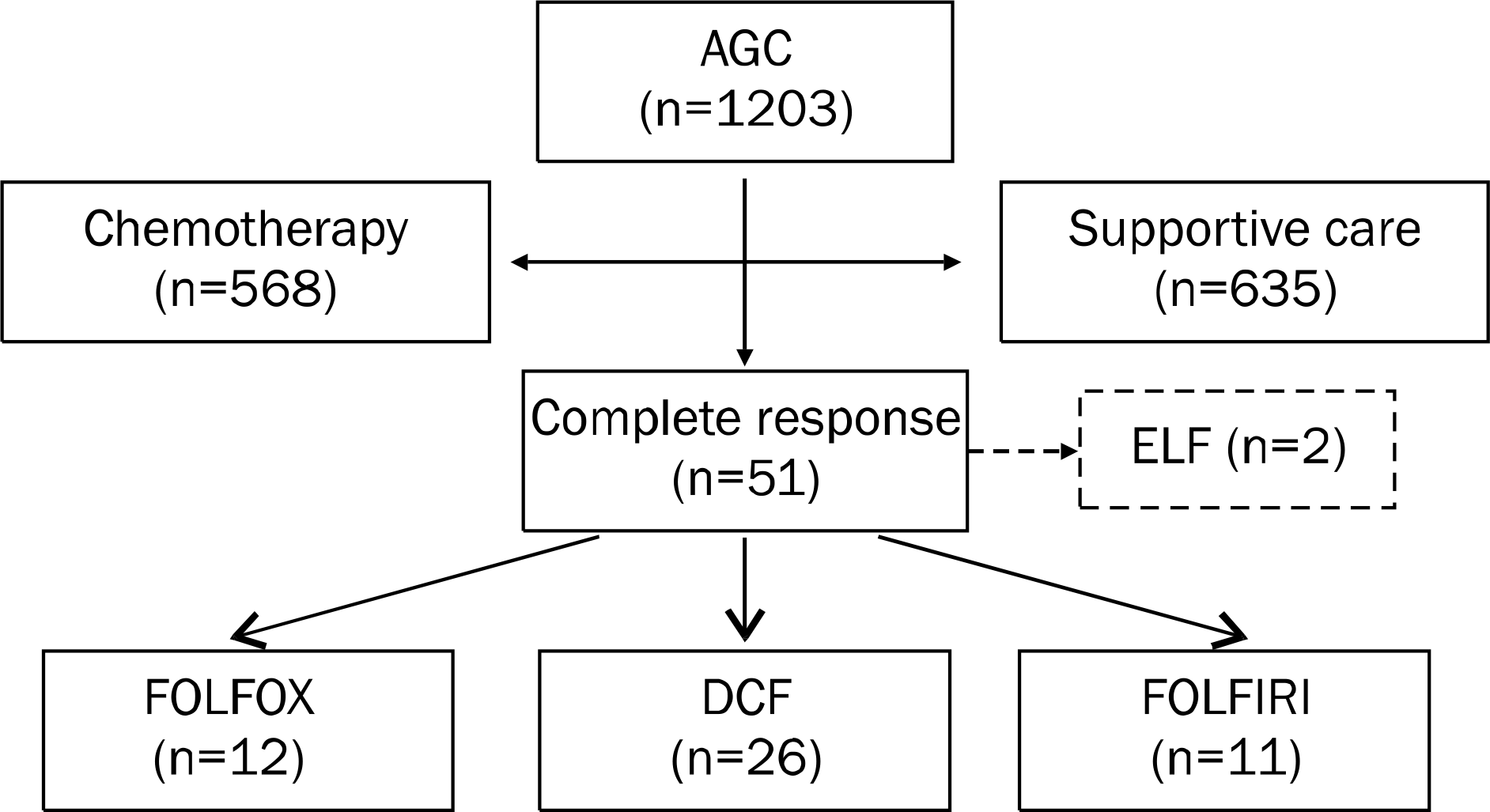 | Fig. 1.Distribution of enrolled patients. Complete response was observed in 51 patients. Of these 51 patients, 2 were treated etoposide, leucovorin and 5-fluorouracil (ELF); therefore, we enrolled the remaining 49 patients who were treated with 5-fluorouracil- leucovorin-oxaliplatin (FOLFOX), docetaxel-cisplatin-5-fluorouracil (DCF) and 5-fluorouracil-leucovorin-irinotecan (FOLFIRI). AGC, advanced gastric cancer. |
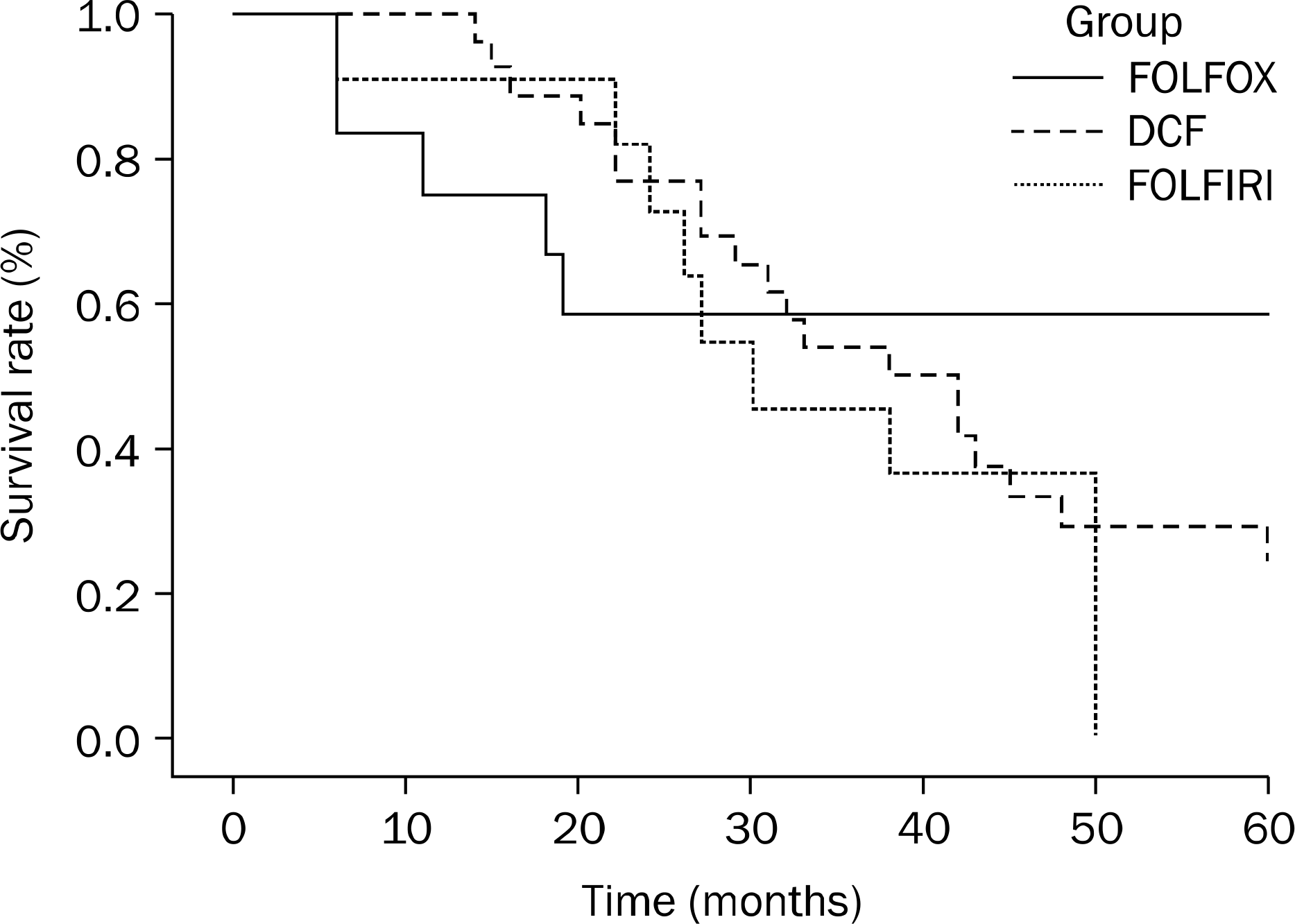 | Fig. 2.Survival rates of patients according to regimens. Statistical difference among in 1-year survival rates (p=0.035), but no difference in 3-year survival rates was observed among the 3 regimen groups. FOLFOX, 5-fluorouracil-leucovorin-oxaliplatin; DCF, docetaxel-cisplatin-5-fluorouracil; FOLFIRI, 5-fluorouracil-leucovorin-irinotecan. |
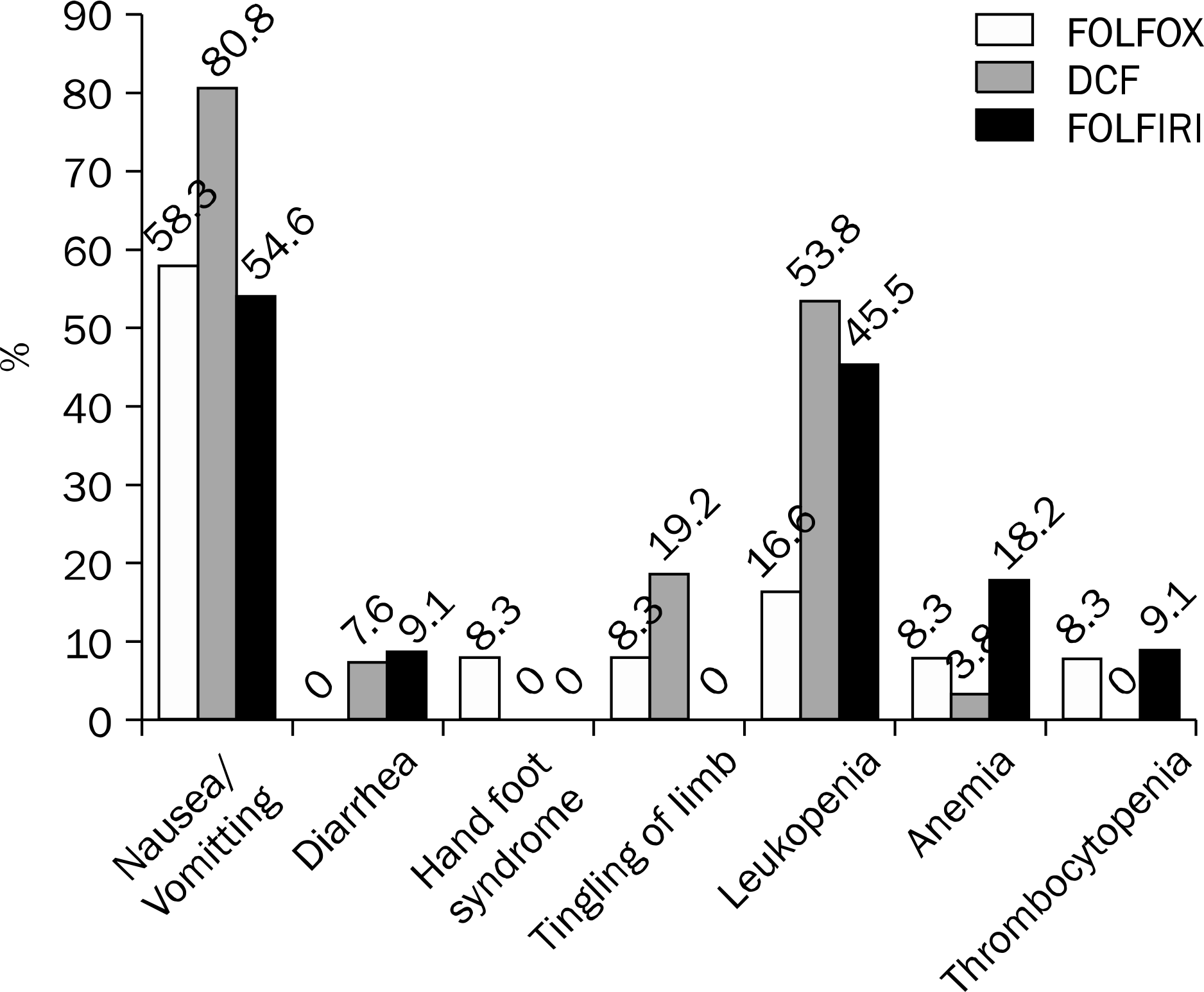 | Fig. 3.Comparison of the toxicities of chemotherapy regimens. No statistical difference was observed among the 3 regimen groups. FOLFOX, 5-fluorouracil-leucovorin-oxaliplatin; DCF, docetaxel-cispla-tin-5-fluorouracil; FOLFIRI, 5-fluorouracil-leucovorin-irinotecan. |
Table 1.
Baseline Characteristics of the Patients with Complete Response
| FOLFOX (n=12) | DCF (n=26) | FOLFIRI (n=11) | p-value | |
|---|---|---|---|---|
| Age (yr) | 51 (41–77) | 58 (35–75) | 51 (37–75) | 0.368 |
| Sex (male: female) | 10:2 | 8:18 | 5:6 | 0.303 |
| Follow-up (months) | 36 (6–74) | 39 (14–86) | 30 (8–50) | |
| Prior surgery | 0.919 | |||
| None | 0 (0.0) | 1 (3.8) | 0 (0.0) | |
| Subtotal | 5 (41.7) | 10 (38.5) | 4 (36.4) | |
| Total | 7 (58.3) | 15 (57.5) | 7 (63.6) | |
| Pathological stage | 0.566 | |||
| Ib | 0 (0.0) | 1 (3.8) | 0 (0.0) | |
| II | 1 (8.3) | 2 (7.7) | 1 (9.1) | |
| IIIa | 2 (16.6) | 3 (11.5) | 2 (18.2) | |
| IIIb | 3 (25.0) | 4 (15.4) | 0 (0.0) | |
| IV | 6 (50.0) | 16 (61.5) | 8 (72.7) | |
| R0/R1/R2 resection | 0.062 | |||
| R0 | 11 | 23 | 6 | |
| R1 | 1 | 3 | 5 | |
| R2 | 0 | 0 | 0 | |
| Borrhman type | 0.331 | |||
| B1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| B2 | 7 (58.3) | 10 (38.5) | 5 (45.5) | |
| B3 | 5 (42.7) | 11 (42.3) | 4 (36.4) | |
| B4 | 0 (0.0) | 5 (19.2) | 2 (18.2) | |
| Location of cancer a | ||||
| Antrum | 3 (25.0) | 10 (38.5) | 2 (18.2) | |
| Body | 7 (58.3) | 17 (65.4) | 3 (27.3) | |
| Cardia | 2 (16.7) | 1 (3.8) | 2 (18.2) | |
| Angle | 2 (16.7) | 3 (11.5) | 1 (9.1) | |
| Histologic classification | ||||
| Adenocarcinoma | 11 | 24 | 9 | |
| Signet ring cell type | 0 | 0 | 1 | |
| Other type | 1 | 2 | 1 | |
| CEA level before Tx | ||||
| Elevation | 1 | 2 | 2 | |
| Normal range | 5 | 17 | 9 | |
| Not checked | 6 | 7 | 0 | |
| Prior chemotherapy | ||||
| Yes | 0 | 0 | 0 | |
| No | 12 | 26 | 11 |
Table 2.
Comparisons of Toxicity according to Chemotherapy Regimens




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download