Abstract
Background/Aims
The aim of this study is to assess serum procalcitonin (PCT) for early prediction of severe acute pancreatitis compared with multiple scoring systems and biomarkers.
Methods
Forty-four patients with acute pancreatitis confirmed by radiological evidences, laboratory assessments, and clinical manifestation were prospectively enrolled. All blood samples and image studies were obtained within 24 hours of admission.
Results
Acute pancreatitis was graded as severe in 19 patients and mild in 25 patients according to the Atlanta criteria. Levels of serum PCT were significantly higher in severe acute pancreatitis (p=0.001). The accuracy of serum PCT as a predicting marker was 77.3%, which was similar to the acute physiology and chronic health examination (APACHE)-II score, worse than the Ranson score (93.2%) and better than the Balthazar CT index (65.9%). The most effective cutoff level of serum PCT was estimated at 1.77 ng/mL (AUC=0.797, 95% CI=0.658-0.935). In comparision to other simple biomarkers, serum PCT had more accurate value (77.3%) than C-reactive protein (68.2%), urea (75.0%) and lactic dehydrogenase (72.7%). Logistic regression analysis revealed that serum PCT has statistical significance in acute severe pancreatitis. Assessment of serum PCT levels and length of hospital stay by simple linear regression analysis revealed effective p-value with low R square level, which could make only possibilty for affection of serum PCT to admission duration (r2=0.127, p=0.021).
References
1. Triester SL, Kowdley KV. Prognostic factors in acute pancreatitis. J Clin Gastroenterol. 2002; 34:167–176.

2. Kusske AM, Rongione AJ, Reber HA. Cytokines and acute pancreatitis. Gastroenterology. 1996; 110:639–642.

3. Larvin M. Circulating mediators in acute pancreatitis as predictors of severity. Scand J Gastroenterol Suppl. 1996; 219:16–19.

4. Windsor AC, Kanwar S, Li AG, et al. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut. 1998; 42:431–435.

5. Al-Nawas B, Krammer I, Shah PM. Procalcitonin in diagnosis of severe infections. Eur J Med Res. 1996; 1:331–333.
6. Kylänpää-Bäck ML, Takala A, Kemppainen E, Puolakkainen P, Haapiainen R, Repo H. Procalcitonin strip test in the early detection of severe acute pancreatitis. Br J Surg. 2001; 88:222–227.

7. Lempinen M, Puolakkainen P, Kemppainen E. Clinical value of severity markers in acute pancreatitis. Scand J Surg. 2005; 94:118–123.

8. Gurda-Duda A, Kuś nierz-Cabala B, Nowak W, Naskalski JW, Kulig J. Assessment of the prognostic value of certain acute-phase proteins and procalcitonin in the prognosis of acute pancreatitis. Pancreas. 2008; 37:449–453.

9. Modrau IS, Floyd AK, Thorlacius-Ussing O. The clinical value of procalcitonin in early assessment of acute pancreatitis. Am J Gastroenterol. 2005; 100:1593–1597.

10. Bumbasirevic V, Radenkovic D, Jankovic Z, et al. Severe acute pancreatitis: overall and early versus late mortality in intensive care units. Pancreas. 2009; 38:122–125.
11. Rau BM, Kemppainen EA, Gumbs AA, et al. Early assessment of pancreatic infections and overall prognosis in severe acute pancreatitis by procalcitonin (PCT): a prospective international multicenter study. Ann Surg. 2007; 245:745–754.
12. Toouli J, Brooke-Smith M, Bassi C, et al. Guidelines for the management of acute pancreatitis. J Gastroenterol Hepatol. 2002; 17(Suppl):S15–S39.

13. Villatoro E, Bassi C, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev. 2006; 4:CD002941.

14. Servín-Torres E, Velázquez-García JA, Delgadillo-Teyer G, Galindo-Mendoza L, Bevia-Pérez F, Rivera-Bennet F. Severe acute pancreatitis: surgical management in a third-level hospital. Cir Cir. 2009; 77:407–410.
15. Dervenis C, Bassi C. Evidence-based assessment of severity and management of acute pancreatitis. Br J Surg. 2000; 87:257–258.

16. Wyncoll DL. The management of severe acute necrotising pancreatitis: an evidence-based review of the literature. Intensive Care Med. 1999; 25:146–156.

17. Neoptolemos JP, Kemppainen EA, Mayer JM, et al. Early prediction of severity in acute pancreatitis by urinary trypsinogen ac-tivation peptide: a multicentre study. Lancet. 2000; 355:1955–1960.

18. Müller CA, Uhl W, Printzen G, et al. Role of procalcitonin and granulocyte colony stimulating factor in the early prediction of in-fected necrosis in severe acute pancreatitis. Gut. 2000; 46:233–238.
19. Puolakkainen P, Valtonen V, Paananen A, Schröder T. C-reactive protein (CRP) and serum phospholipase A2 in the assessment of the severity of acute pancreatitis. Gut. 1987; 28:764–771.

20. Wilson C, Heads A, Shenkin A, Imrie CW. C-reactive protein, anti-proteases and complement factors as objective markers of severity in acute pancreatitis. Br J Surg. 1989; 76:177–181.

21. Chen CC, Wang SS, Lee FY, Chang FY, Lee SD. Proinflammatory cytokines in early assessment of the prognosis of acute pancreatitis. Am J Gastroenterol. 1999; 94:213–218.

22. Gendrel D, Bohuon C. Procalcitonin as a marker of bacterial infection. Pediatr Infect Dis J. 2000; 19:679–687.

23. Braithwaite S. Procalcitonin: new insights on regulation and origin. Crit Care Med. 2000; 28:586–588.

24. Mándi Y, Farkas G, Takács T, Boda K, Lonovics J. Diagnostic relevance of procalcitonin, IL-6, and sICAM-1 in the prediction of in-fected necrosis in acute pancreatitis. Int J Pancreatol. 2000; 28:41–49.

25. Riché FC, Cholley BP, Laisné MJ, et al. Inflammatory cytokines, C reactive protein, and procalcitonin as early predictors of necrosis infection in acute necrotizing pancreatitis. Surgery. 2003; 133:257–262.

26. Oláh A, Belágyi T, Issekutz A, Makay R, Zaborszky A. Value of procalcitonin quick test in the differentiation between sterile and in-fected forms of acute pancreatitis. Hepatogastroenterology. 2005; 52:243–245.
27. Wanner GA, Keel M, Steckholzer U, Beier W, Stocker R, Ertel W. Relationship between procalcitonin plasma levels and severity of injury, sepsis, organ failure, and mortality in injured patients. Crit Care Med. 2000; 28:950–957.

28. Ammori BJ, Becker KL, Kite P, et al. Calcitonin precursors in the prediction of severity of acute pancreatitis on the day of admission. Br J Surg. 2003; 90:197–204.

29. Shafiq N, Malhotra S, Bhasin DK, Rana S, Siddhu S, Pandhi P. Estimating the diagnostic accuracy of procalcitonin as a marker of the severity of acute pancreatitis: a meta-analytic approach. JOP. 2005; 6:231–237.
30. Melzi D'Eril GV, Merlini G, Finazzi S, Bosoni T, Barakat B, Pezzilli R. Procalcitonin is not a reliable marker for the assessment of severity in acute pancreatitis without infectious complications. Clin Chem. 2000; 46:428–430.
Fig. 1.
Mean value of serum levels of procalcitonin in mild and severe acute pancreatitis clossified by Atlanta criteria. Levels of serum procalcitonin were significantly higher in severe acute pancreatitis. Md, mild acute pancreatitis (n=25, SD=4.614); Severe, severe acute pancreatitis (n=24, SD= 53.433). ∗Mann-Whitney U test, p=0.001.
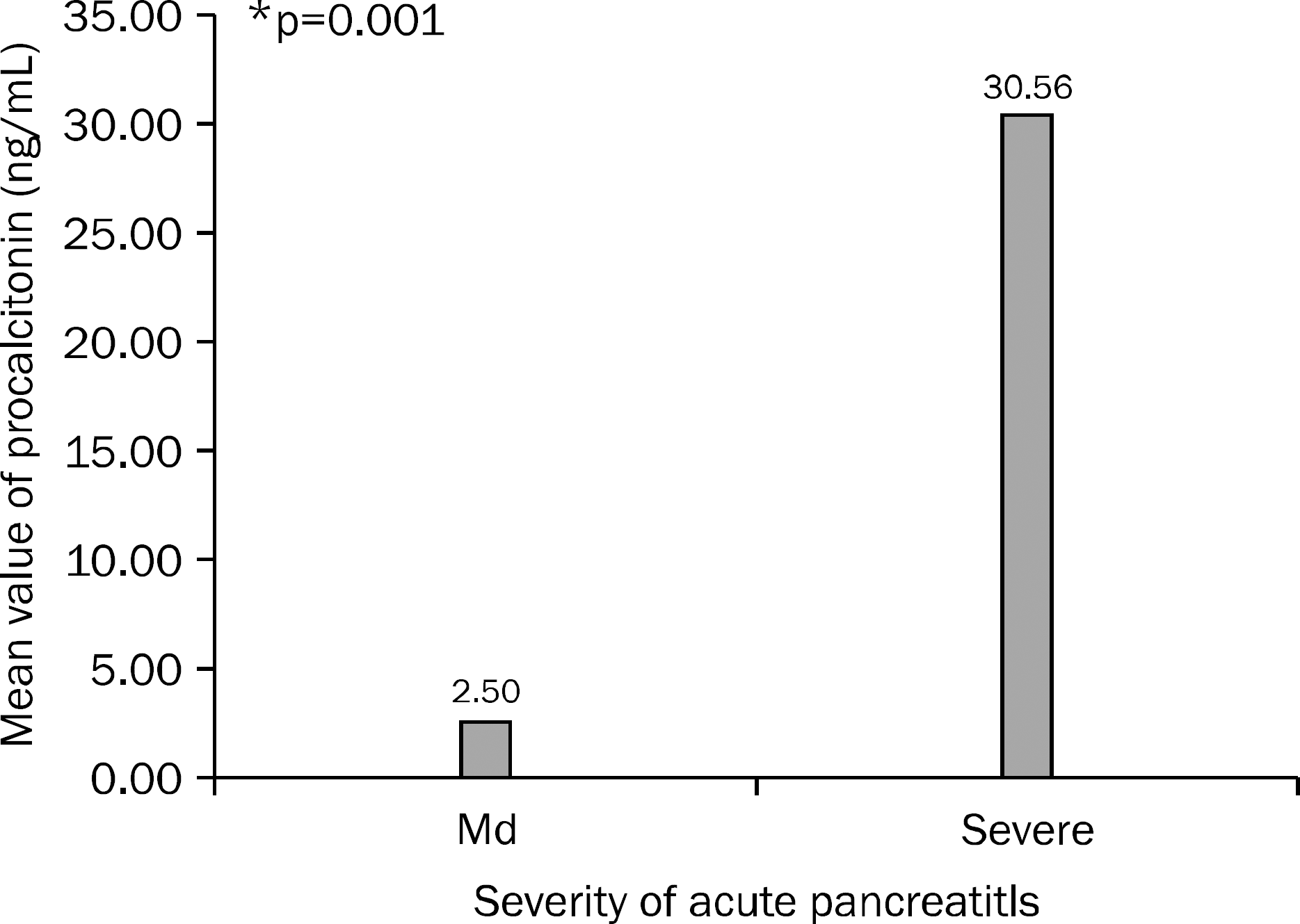
Fig. 2.
Receiver operation characteristic curve of serum levels of procalcitonin in prediction of severity of acute pancreatitis. Numbers of observation=44. AUC, area under curve.
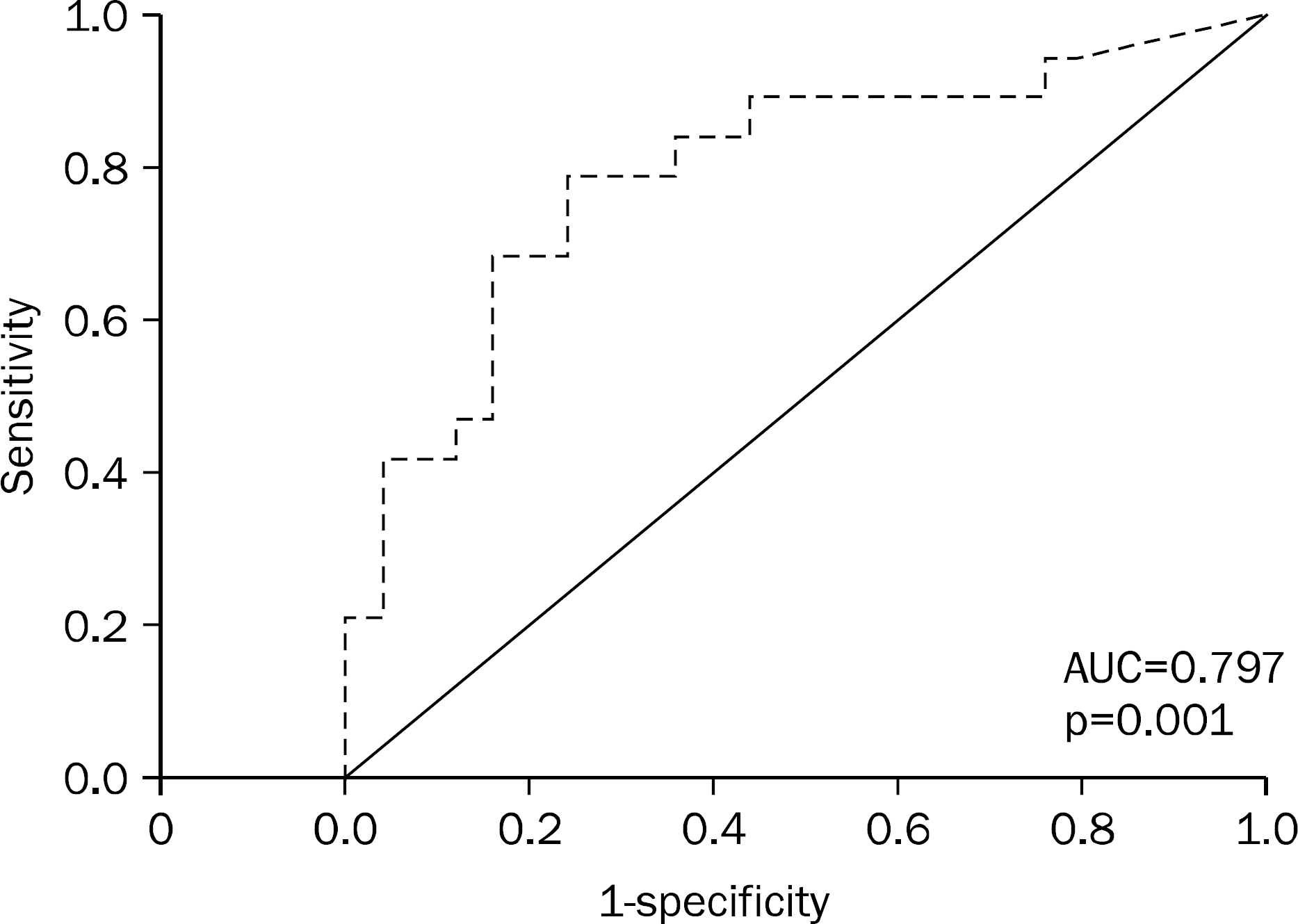
Fig. 3.
Correlation between serum procalcitonin levels and duration of admission in patients with acute pancreatitis. Procalcitonin is indendent variable (x), admission duration is dependent variable (y). Coefficient B is −8.791. Coefficient of determinant (r2)=0.127, t value=2.413, dependent p-value=0.021.
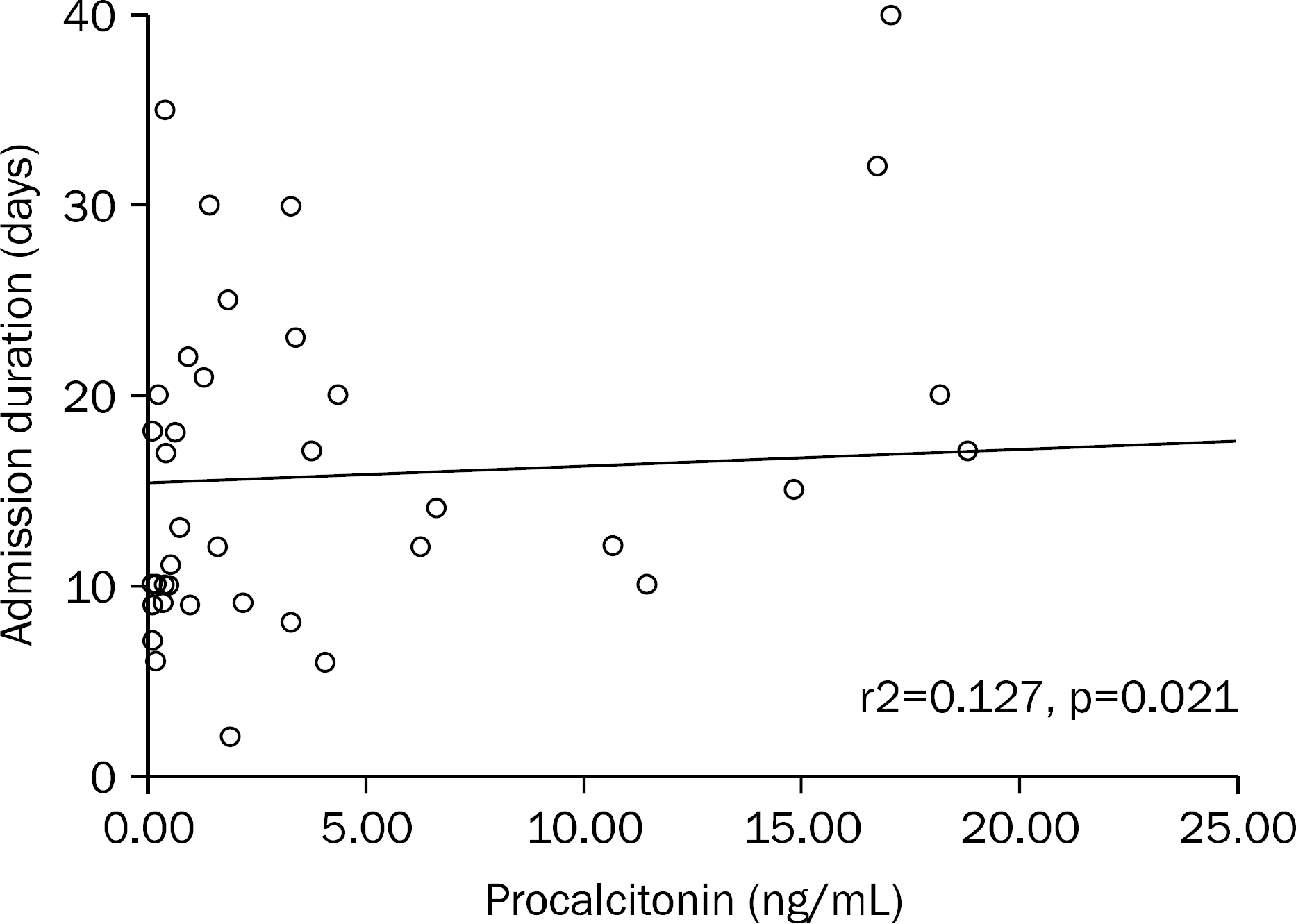
Table 1.
Characteristics of Patients with Acute Pancreatitis
Table 2.
Analysis of Acute Pancreatitis Parameters
Table 3.
Serum Procalcitonin in Prediction of Severe Acute Pancreatitis
Table 4.
Logistic Regression Analysis of Risk Factors for Severe Acute Pancreatitis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download