Abstract
Background/Aims
In the Helicobacter pylori (H. Pylori)-negative normal stomach, collecting venules are visible over all the gastric body as numerous minute points evaluated with standard endoscopy. This finding was termed regular arrangement of collecting venules (RAC), and its absence suggests H. pylori gastritis. The aim of this study was to evaluate the correlation between the RAC and rapid urease test.
Methods
Two hundred sixty three consecutive adults undergoing upper digestive endoscopy and rapid urease test were included. The lesser curvature of the lower corpus was evaluated for the RAC pattern using a standard endoscope and different hemoglobin index. Two biopsies from the lesser curvature of the antrum and the greater curvature of the body were collected for rapid urease test.
Results
H. pylori were detected in 51.3% (135/263) patients. Of the 57 patients with H. pylori-negative normal stomachs 53 patients (93%) had RAC. As a determinant of the normal stomach without H. pylori infection, the presence of RAC had 41.4% sensitivity, 97.0% specificity, 93.0% positive predictive value and 63.6% negative predictive value.
Go to : 
References
1. Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984; 1:1311–1315.

2. Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991; 325:1127–1131.
3. Forman D, Newell DG, Fullerton F, et al. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ. 1991; 302:1302–1305.
4. Yim JY, Choi SH, Park MJ, et al. Seroprevalence of Helicobacter pylori in health check-up subjects. Korean J Med. 2006; 70:636–642.
5. Kim N, Kim JJ, Choe YH, Kim HS, Kim JI, Chung IS. Korean College of Helicobacter and Upper Gastrointestinal Research; Korean Association of Gastroenterology. Diagnosis and treatment guidelines for helicobacter pylori infection in Korea. Korean J Gastroenterol. 2009; 54:269–278.
6. Redéen S, Petersson F, Jönsson KA, Borch K. Relationship of gastroscopic features to histological findings in gastritis and Helicobacter pylori infection in a general population sample. Endoscopy. 2003; 35:946–950.
7. Hassall E, Dimmick JE. Unique features of Helicobacter pylori disease in children. Dig Dis Sci. 1991; 36:417–423.
8. Belair PA, Metz DC, Faigel DO, Furth EE. Receiver operator characteristic analysis of endoscopy as a test for gastritis. Dig Dis Sci. 1997; 42:2227–2233.
9. Laine L, Cohen H, Sloane R, Marin-Sorensen M, Weinstein WM. Interobserver agreement and predictive value of endoscopic findings for H. pylori and gastritis in normal volunteers. Gastrointest Endosc. 1995; 42:420–423.
10. Tytgat GN. The Sydney System: endoscopic division. Endoscopic appearances in gastritis/duodenitis. J Gastroenterol Hepatol. 1991; 6:223–234.

11. Khakoo SI, Lobo AJ, Shepherd NA, Wilkinson SP. Histological assessment of the Sydney classification of endoscopic gastritis. Gut. 1994; 35:1172–1175.

12. Yagi K, Nakamura A, Sekine A. Characteristic endoscopic and magnified endoscopic findings in the normal stomach without Helicobacter pylori infection. J Gastroenterol Hepatol. 2002; 17:39–45.
13. Yagi K, Nakamura A, Sekine A. Comparison between magnifying endoscopy and histological, culture and urease test findings from the gastric mucosa of the corpus. Endoscopy. 2002; 34:376–381.

14. Nakayama Y, Horiuchi A, Kumagai T, et al. Discrimination of normal gastric mucosa from Helicobacter pylori gastritis using standard endoscopes and a single observation site: studies in children and young adults. Helicobacter. 2004; 9:95–99.
15. Mond DJ, Pochaczevsky R, Vernace F, Bank S, Chow KW. Can the radiologist recognize Helicobacter pylori gastritis? J Clin Gastroenterol. 1995; 20:199–202.
16. Nakagawa S. Relationship between histological gastritis and mucosal microvascular observations using magnifying endoscopy. Hokkaido Igaku Zasshi. 2003; 78:349–356.
17. Yagi K, Aruga Y, Nakamura A, Sekine A. Regular arrangement of collecting venules (RAC): a characteristic endoscopic feature of Helicobacter pylori-negative normal stomach and its relationship with esophagogastric adenocarcinoma. J Gastroenterol. 2005; 40:443–452.

18. Machado RS, Viriato A, Kawakami E, Patrício FR. The regular arrangement of collecting venules pattern evaluated by standard endoscope and the absence of antrum nodularity are highly indicative of Helicobacter pylori uninfected gastric mucosa. Dig Liver Dis. 2008; 40:68–72.
19. Yang JM, Chen L, Fan YL, Li XH, Yu X, Fang DC. Endoscopic patterns of gastric mucosa and its clinicopathological significance. World J Gastroenterol. 2003; 9:2552–2556.

20. Yan SL, Wu ST, Chen CH, et al. Mucosal patterns of Helicobacter pylori-related gastritis without atrophy in the gastric corpus using standard endoscopy. World J Gastroenterol. 2010; 16:496–500.
21. Midolo P, Marshall BJ. Accurate diagnosis of Helicobacter pylori. Urease tests. Gastroenterol Clin North Am. 2000; 29:871–878.
22. Lim LL, Ho KY, Ho B, Salto-Tellez M. Effect of biopsies on sensitivity and specificity of ultra-rapid urease test for detection of Helicobacter pylori infection: a prospective evaluation. World J Gastroenterol. 2004; 10:1907–1910.
23. Weck MN, Brenner H. Association of Helicobacter pylori infection with chronic atrophic gastritis: Meta-analyses according to type of disease definition. Int J Cancer. 2008; 123:874–881.
24. Weck MN, Gao L, Brenner H. Helicobacter pylori infection and chronic atrophic gastritis: associations according to severity of disease. Epidemiology. 2009; 20:569–574.
Go to : 
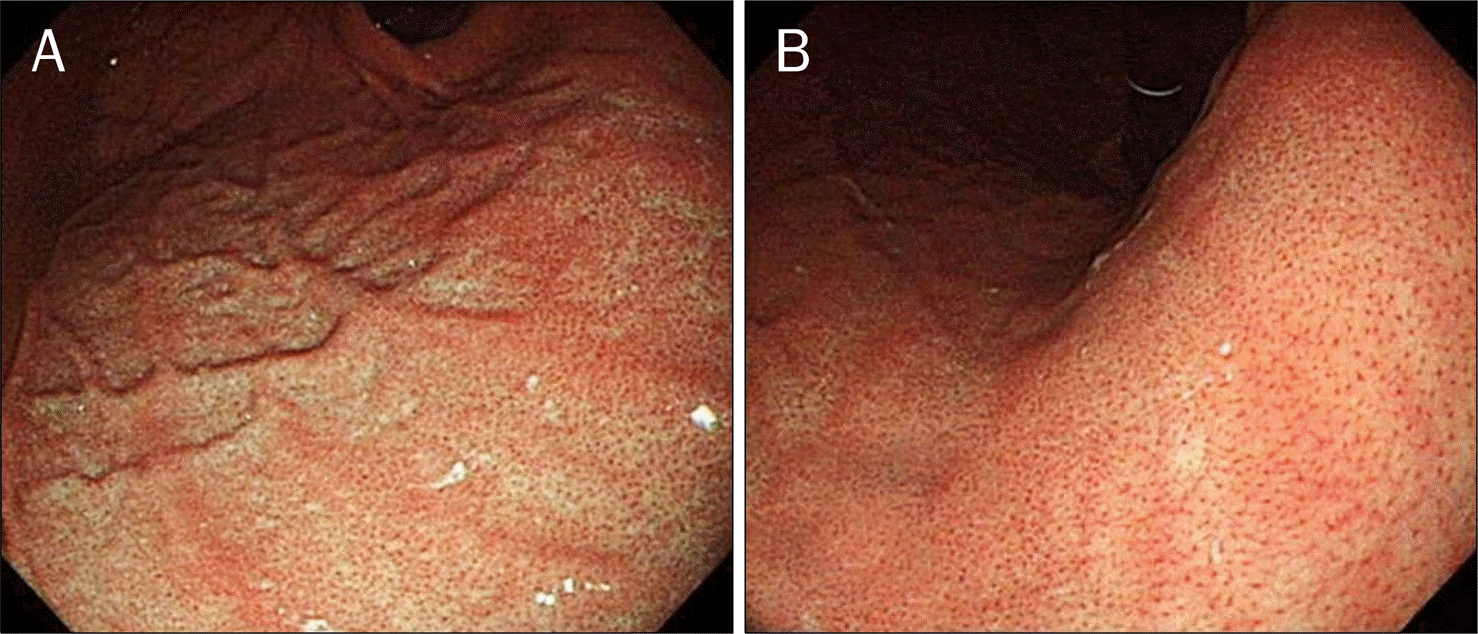 | Fig. 1.Typical image of a regular arrangement of collecting venule (RAC) positive case. (A) Typical close observation view of a RAC-positive case. Numerous minute red points of almost similar size were seen at regular intervals all over the monitor view at the lesser curvature of the lower corpus. (B) Endoscopic image of gastric mucosa switching hemoglobin index in RAC-positive case. |
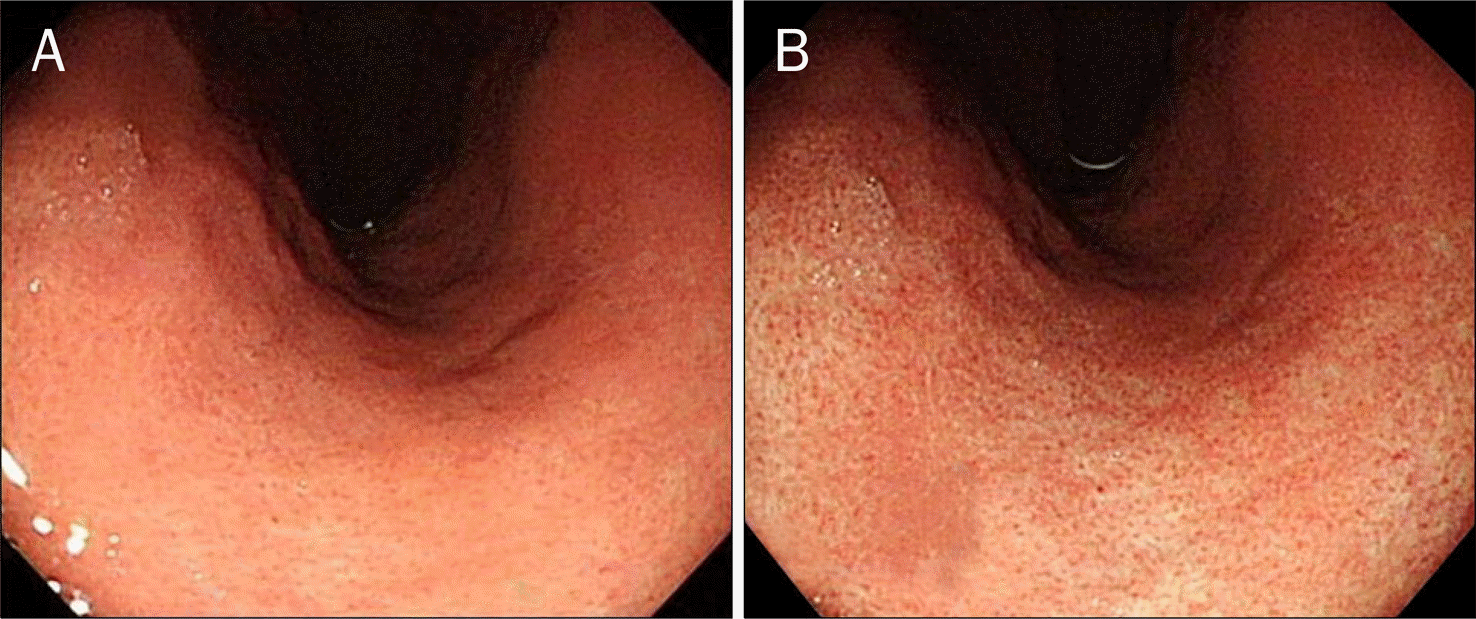 | Fig. 2.Typical image of a regular arrangement of collecting venule (RAC) negative case. (A) Typical close observation view of a RAC-negative case. The red points were irregularly presented or absent. (B) Endoscopic image of gastric mucosa switching hemoglobin index in RAC-negative case. |
Table 1.
Baseline Characteristics of the 263 Patients
Table 2.
Correlation between RAC and Rapid Urease Test
| Variables | Rapid urease test, n | Total, n | |
|---|---|---|---|
| Negative | Positive | ||
| RAC-positive | 53 | 4 | 57 |
| RAC-negative | 75 | 131 | 206 |
| Total | 128 | 135 | 263 |
Table 3.
Comparison of Sensitivity, Specificity, PPV, NPV, and Accuracy of RAC Classified by the Presence or Absence of Atrophic Gastritis
Table 4.
Comparison of Sensitivity, Specificity, PPV, NPV, and Accuracy of RAC according to Age




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download