Abstract
Background/Aims
Sustained HBV DNA reduction is necessary for biochemical remission, histological improvement, and prevention of complications. We analyzed the time taken from HBV DNA loss to viral breakthrough after antiviral treatment in patients with chronic hepatitis B (CHB). The early fall of the HBV DNA level to undetectable levels assessed really whether it is related to late breakthrough.
Methods
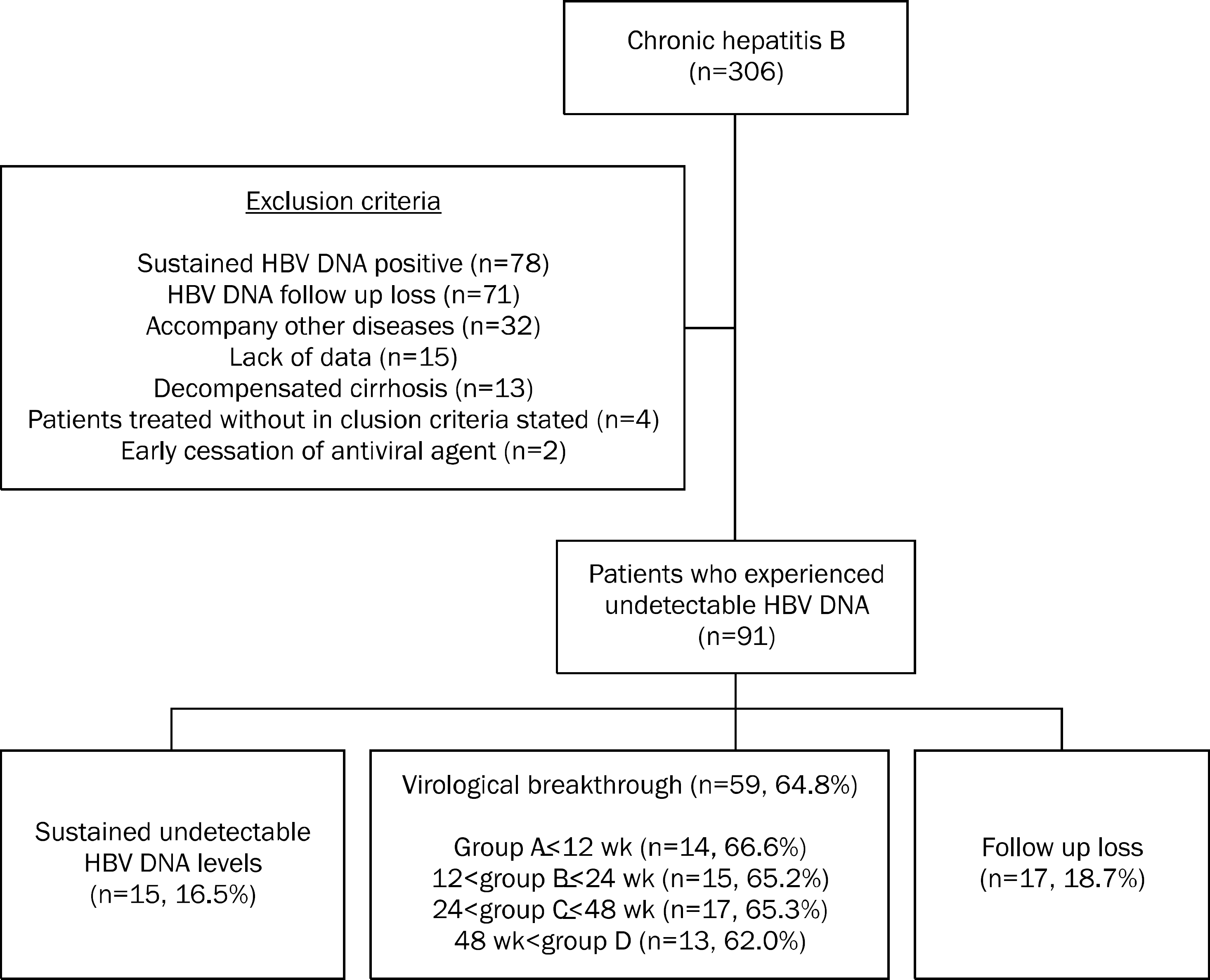
A total of 91 patients whose HBV DNA levels dropped below undetectable levels were chosen from lamivudine-treated 306 patients and were analyzed retrospectively. The patients were divided into 4 groups (A≤12, 12< B≤24, 24< C≤48, D>48 wk) according to the time taken for the HBV DNA to decrease below undetectable levels. HBV DNA level was determined every 3 months.
Results
The mean time taken for loss of HBV DNA was 34±28 wk. The baseline ALT differed significantly among groups (A: 382±274, B: 340±30, C: 166±92, D: 54±100 IU/L) (p=0.007). Fifty nine of the 91 patients (64.8%) experienced viral breakthrough. The mean interval between HBV DNA loss and viral breakthrough was 65±40 wk and differed significantly between group A, B (82±43 wk) and group C, D (56±28 wk) (p=0.015). In multivariate analysis, only HBV DNA loss within 24 wk, was found to be independently associated with late viral breakthrough (p=0.035). Undetectable HBV DNA after 24 wk was associated with high odd ratio of 3.24 (95% CI, 1.09-9.67).
Go to : 
References
1. Moucari R, Korevaar A, Lada O, et al. High rates of HBsAg sero-conversion in HBeAg-positive chronic hepatitis B patients re-sponding to interferon: a long-term follow-up study. J Hepatol. 2009; 50:1084–1092.

2. Liaw YF, Gane E, Leung N, et al. 2-year GLOBE trial results: telbi-vudine Is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009; 136:486–495.

3. Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008; 48:335–352.

4. European Association For The Study Of The Liver. EASL clinical practice guidelines: management of chronic hepatitis B. J Hepatol. 2009; 50:227–242.
5. Chang TT, Suh DJ. Current approaches for treating chronic hepatitis B: when to start, what to start with, and when to stop. Hepatol Int. 2008; 2(Suppl 1):19–27.

7. Liaw YF. Hepatitis B virus replication and liver disease progression: the impact of antiviral therapy. Antivir Ther. 2006; 11:669–679.
8. Keeffe EB, Zeuzem S, Koff RS, et al. Report of an international workshop: Roadmap for management of patients receiving oral therapy for chronic hepatitis B. Clin Gastroenterol Hepatol. 2007; 5:890–897.

9. Yao G. Entecavir is a potent anti-HBV drug superior to lamivudine: experience from clinical trials in China. J Antimicrob Chemother. 2007; 60:201–205.

10. Liaw YF, Leung N, Kao JH, et al. Asian-Pacific consensus state-ment on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008; 2:263–283.

11. Gallego A, Sheldon J, García-Samaniego J, et al. Evaluation of initial virological response to adefovir and development of adefovir-resistant mutations in patients with chronic hepatitis B. J Viral Hepat. 2008; 15:392–398.

12. Liu CJ, Kao JH, Chen DS. Therapeutic implications of hepatitis B virus genotypes. Liver Int. 2005; 25:1097–1107.

13. Liaw YF, Leung N, Guan R, et al. Asian-Pacific consensus state-ment on the management of chronic hepatitis B: a 2005 update. Liver Int. 2005; 25:472–489.

14. Kim SS, Chung MG, Ju KT, et al. Clinical and virologic characteristics of lamivudine resistance in HBV-associated chronic liver disease. Korean J Hepatol. 2002; 8:405–417.
Go to : 
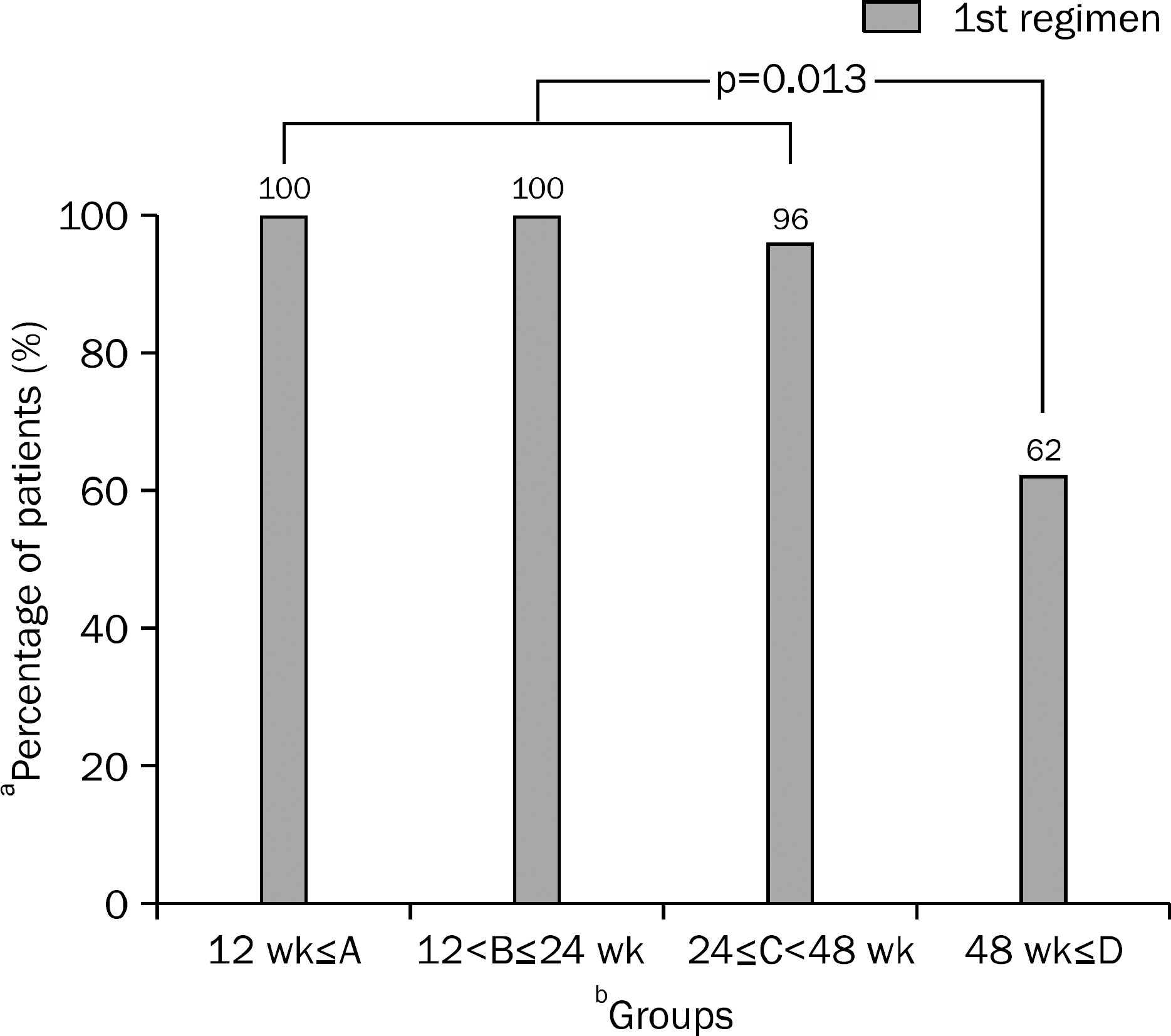 | Fig. 2.Initial lamivudine treatment or change in regimen compared with time to undetectable HBV DNA. Twenty one of the 21 patients (100%), 23 of the 23 patients, (100%), 25 of the 26 patients (96%),13 of the 21 patients (62%) had undetectable viral loads without change of initial antiviral agent and there was a significant difference between groups A, B, C and group D (p=0.013).
a Percentage of patients without change of initial lamivudine regimen.
b Groups according to the time to undetectable HBV DNA.
|
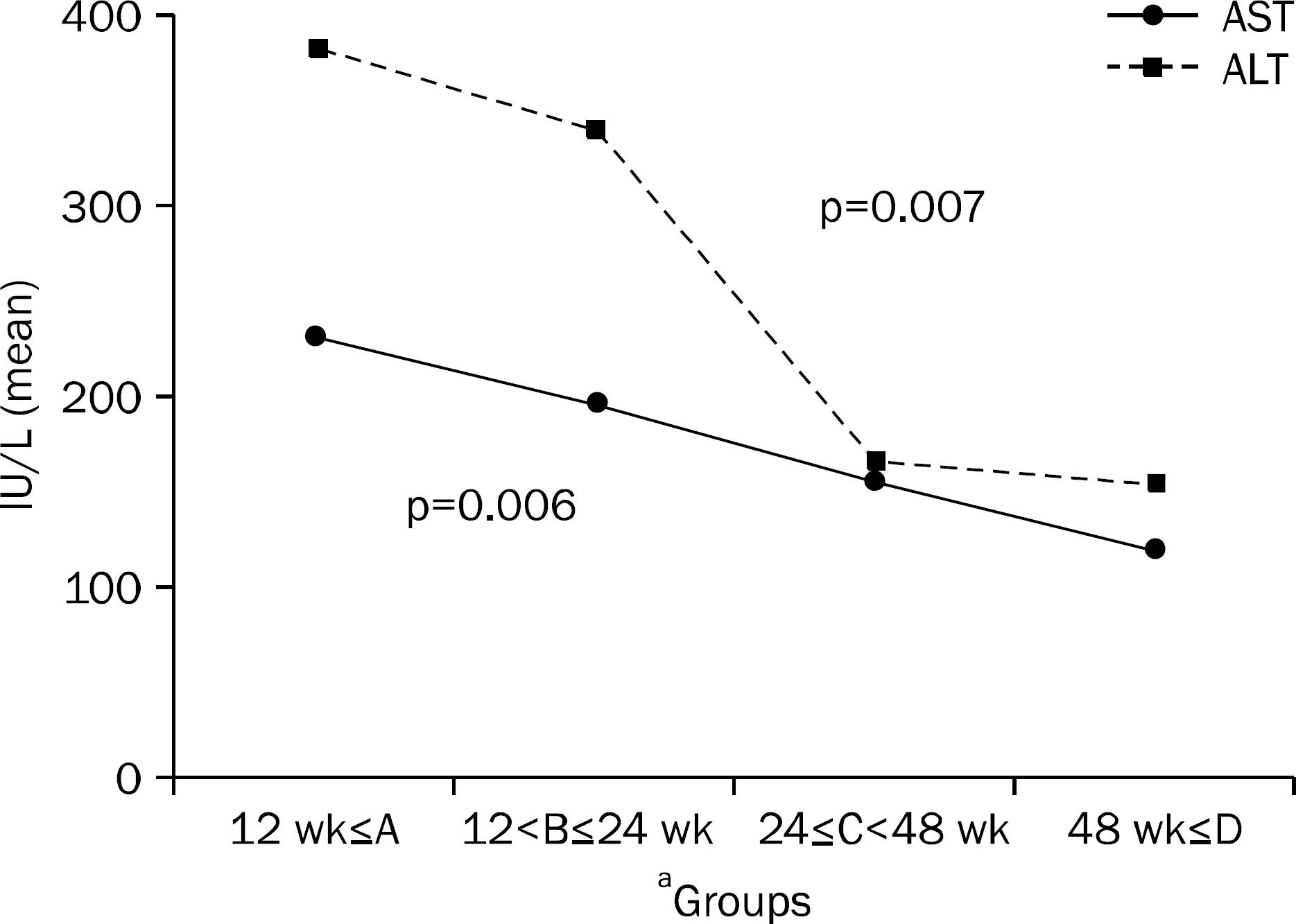 | Fig. 3.Comparison of baseline AST/ALT levels among 4 groups. Baseline AST/ALT levels showed 232±195/382±274 IU/L in group A, 197±121/340±306 IU/L in group B, 156±144/166±92 IU/L in group C and 119±89/154±100 IU/L in group D and showed a significant difference between each group (p=0.006/p=0.007).
a Groups according to the time to undetectable HBV DNA
|
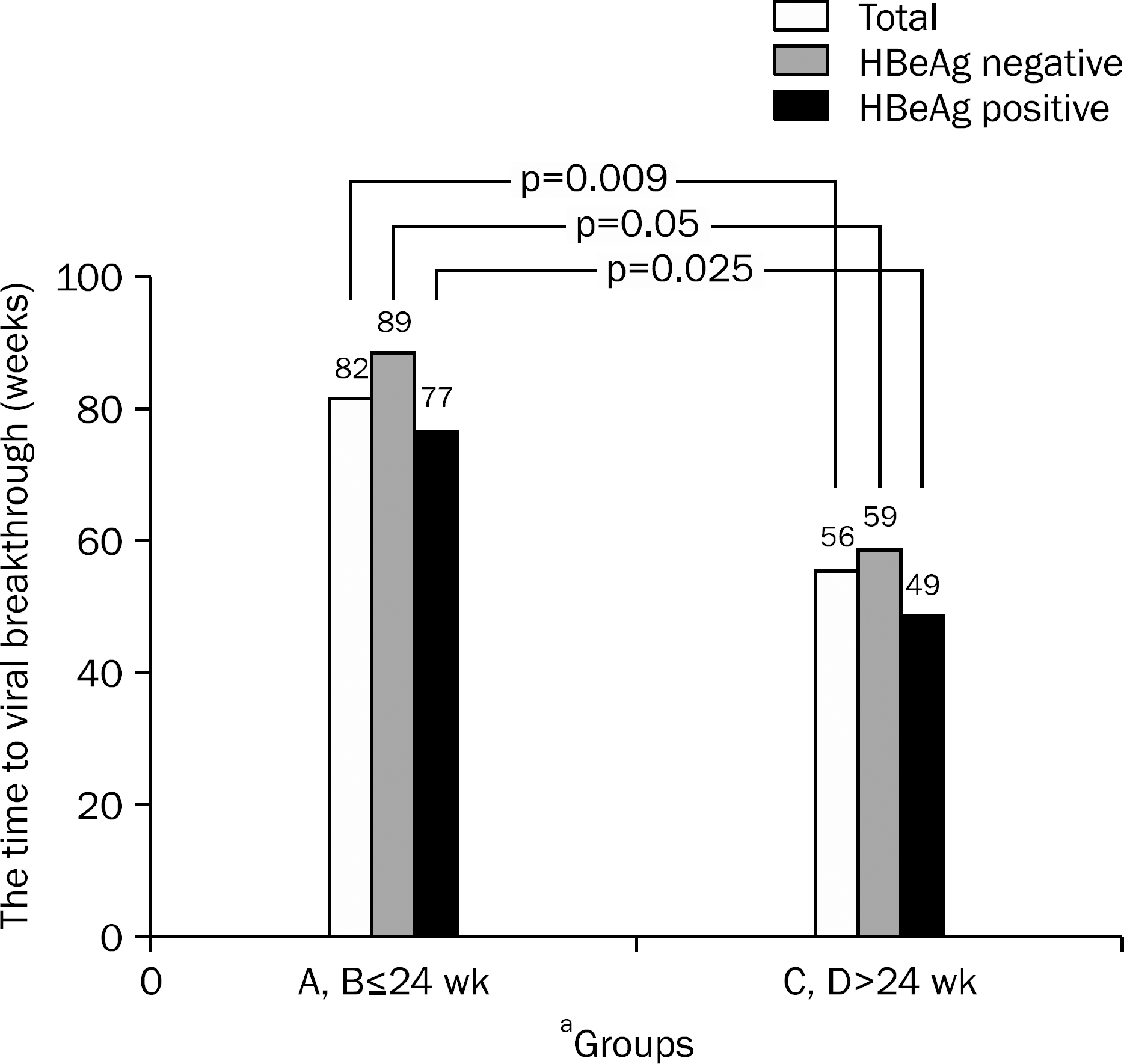 | Fig. 4.The time taken until viral breakthrough according to pretreatment HBeAg status. In HBeAg positive, the mean time period between HBV DNA loss and viral breakthrough was 77±44 wk in groups A and B and 49±19 wk in groups C and D (p=0.025). In HBeAg negative, the mean time period between HBV DNA loss and viral breakthrough was 89±43 wk in groups A and B and 59±22 wk in groups C and D (p=0.05).
a Groups according to the time to undetectable HBV DNA.
|
Table 1.
Characteristics of the Patients with Undetectable HBV DNA
Table 2.
Baseline Characteristics between 4 Groups (A≤12, 12<B ≤24, 24<C≤48, and D>48 wk)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download