Abstract
Background/Aims
Revaprazan (RevanexⓇ) is a novel proton pump inhibitor (PPI) that has a somewhat different effect on proton pump compared with the other PPI's, also (called as ‘acid pump antagonist'). We aimed to examine the false negative rate of 13 C-urea breath test (UBT) in the patients with Helicobacter pylori (H. pylori) associated peptic ulcer disease who were treated with revaprazan and evaluate the anti-urease activity of revaprazan.
Methods
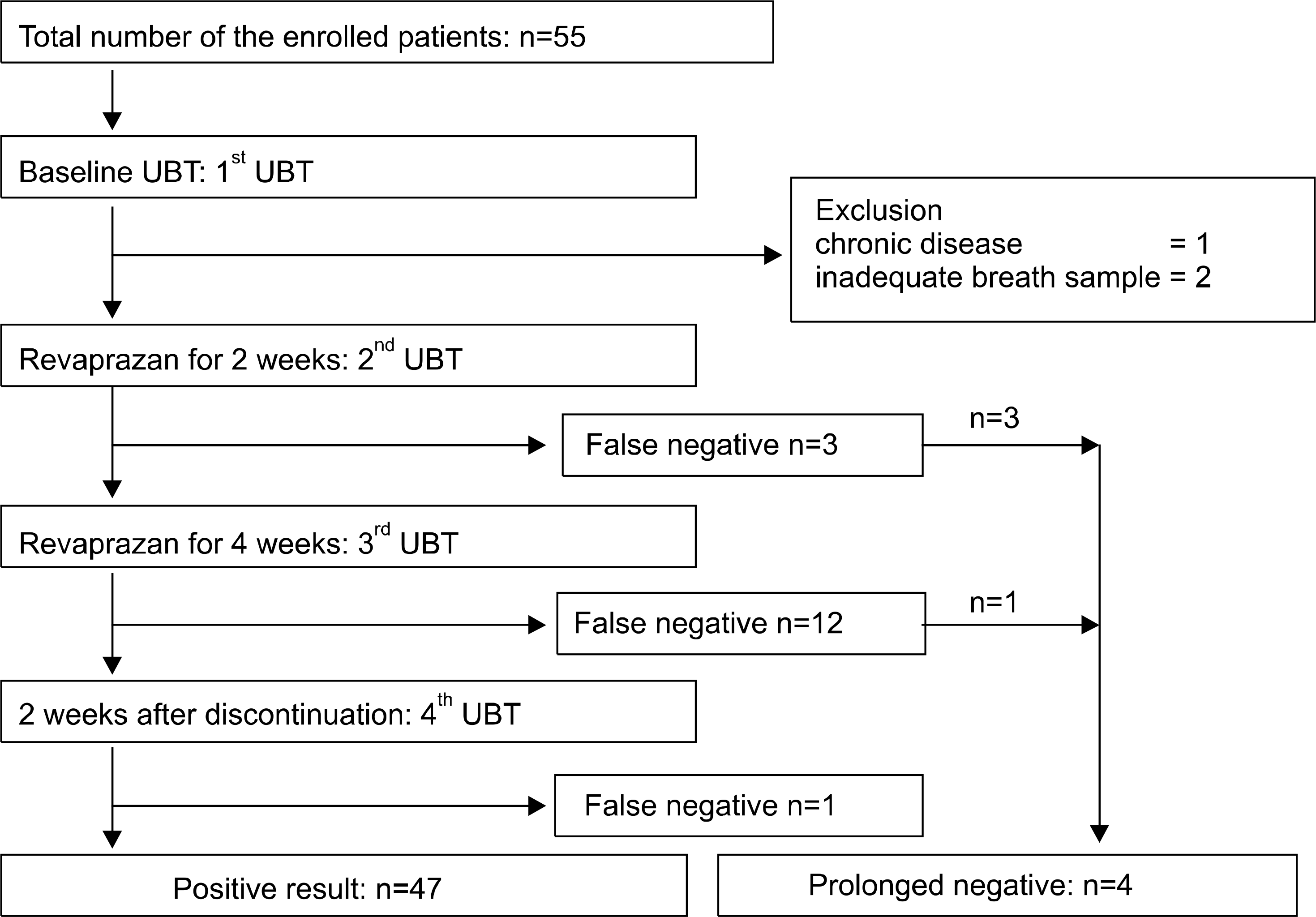
Total 55 patients were enrolled in this study. They received EGD examination between January 2009 and December 2009 and diagnosed histologically as H. pylori associated peptic ulcer disease. All patients took revaprazan only. Three patients were excluded because of underlying chronic disease and in-appropriate breath sampling. The remaining 52 patients had UBT at 0, 2 and 4 weeks of revaprazan use. After 2 weeks of the cessation of revaprazan, they had the fourth UBT.
Results
At 2 and 4 weeks, the false negative rates of UBT were 5.8% and 23.1%, respectively (p=0.05). After 2 weeks of the cessation, the cases of the false negative result were five. Four out of five patients had prolonged negative results on two or three successive tests, and baseline 13 C difference value did not predict the false negative results.
References
1. Yeo M, Kim DK, Chung IS, Moon BS, Song KS, Hahm KB. The novel acid pump antagonists for anti-secretory actions with their peculiar applications beyond acid suppression. J Clin Biochem Nutr. 2006; 38:1–8.

2. Park S, Lee SM, Song K, Lee B, Kang H, Kang J. The pharmacological properties of a novel acid pump antagonist, YH1885. Gut. 2003; 52(Suppl VI):A62.
3. Yu KS, Bae KS, Shon JH, et al. Pharmacokinetic and pharmacodynamic evaluation of a novel proton pump inhibitor, YH1885, in healthy volunteers. J Clin Pharmacol. 2004; 44:73–82.

4. Pope AJ, Boehm MK, Leach C, Ife RJ, Keeling D, Parsons ME. Properties of the reversible K(+)-competitive inhibitor of the gastric (H+/K+)-ATPase, SK&F 97574. I. In vitro activity. Biochem Pharmacol. 1995; 50:1543–1549.
5. Logan RP, Walker MM, Misiewicz JJ, Gummett PA, Karim QN, Baron JH. Changes in the intragastric distribution of Helicobacter pylori during treatment with omeprazole. Gut. 1995; 36:12–16.
6. Stoschus B, Domínguez-Muñoz JE, Kalhori N, Sauerbruch T, Malfertheiner P. Effect of omeprazole on Helicobacter pylori urease activity in vivo. Eur J Gastroenterol Hepatol. 1996; 8:811–813.
7. Laine L, Estrada R, Trujillo M, Knigge K, Fennerty MB. Effect of proton-pump inhibitor therapy on diagnostic testing for Helicobacter pylori. Ann Intern Med. 1998; 129:547–550.
8. Graham DY, Opekun AR, Hammoud F, et al. Studies regarding the mechanism of false negative urea breath tests with proton pump inhibitors. Am J Gastroenterol. 2003; 98:1005–1009.

9. Bravo LE, Realpe JL, Campo C, Mera R, Correa P. Effects of acid suppression and bismuth medications on the performance of diagnostic tests for Helicobacter pylori infection. Am J Gastroenterol. 1999; 94:2380–2383.
10. Savarino V, Bisso G, Pivari M, et al. Effect of gastric acid suppression on 13C-urea breath test: comparison of ranitidine with omeprazole. Aliment Pharmacol Ther. 2000; 14:291–297.

11. Parente F, Sainaghi M, Sangaletti O, et al. Different effects of short-term omeprazole, lansoprazole or pantoprazole on the accuracy of the (13)C-urea breath test. Aliment Pharmacol Ther. 2002; 16:553–557.

12. Dulbecco P, Gambaro C, Bilardi C, et al. Impact of long-term ranitidine and pantoprazole on accuracy of 13C-urea breath test. Dig Dis Sci. 2003; 48:315–321.
13. Adachi K, Fujishiro H, Mihara T, Komazawa Y, Kinoshita Y. Influence of lansoprazole, famotidine, roxatidine and rebamipide administration on the urea breath test for the diagnosis of Helicobacter pylori infection. J Gastroenterol Hepatol. 2003; 18:168–171.
14. Murakami K, Sato R, Okimoto T, et al. Influence of anti-ulcer drugs used in Japan on the result of (13)C-urea breath test for the diagnosis of Helicobacter pylori infection. J Gastroenterol. 2003; 38:937–941.
15. Gatta L, Vakil N, Ricci C, et al. Effect of proton pump inhibitors and antacid therapy on C-urea breath tests and stool test for Helicobacter pylori infection. Am J Gastroenterol. 2004; 99:823–829.
16. Gisbert JP, Pajares JM. 13C-urea breath test in the management of Helicobacter pylori infection. Dig Liver Dis. 2005; 37:899–906.
17. Parente F, Sainaghi M, Sangaletti O, et al. Different effects of short-term omeprazole, lansoprazole or pantoprazole on the accuracy of the (13)C-urea breath test. Aliment Pharmacol Ther. 2002; 16:553–557.

18. Epple HJ, Kirstein FW, Bojarski C, et al. 13C-urea breath test in Helicobacter pylori diagnosis and eradication. Correlation to histology, origin of ‘false’ results, and influence of food intake. Scand J Gastroenterol. 1997; 32:308–314.
19. Mana F, Van Laer W, Bossuyt A, Urbain D. The early effect of proton pump inhibitor therapy on the accuracy of the 13C-urea breath test. Dig Liver Dis. 2005; 37:28–32.

20. Lodato F, Azzaroli F, Turco L, et al. Adverse effects of proton pump inhibitors. Best Pract Res Clin Gastroenterol. 2010; 24:193–201.

21. Laine L, Ahnen D, McClain C, Solcia E, Walsh JH. Review article: potential gastrointestinal effects of long-term acid suppression with proton pump inhibitors. Aliment Pharmacol Ther. 2000; 14:651–668.

22. Gillen D, McColl KE. Problems associated with the clinical use of proton pump inhibitors. Pharmacol Toxicol. 2001; 89:281–286.

23. Borch K, Renvall H, Liedberg G. Gastric endocrine cell hyperplasia and carcinoid tumors in pernicious anemia. Gastroenterology. 1985; 88:638–648.

24. Bardram L, Thomsen P, Stadil F. Gastric endocrine cells in omeprazole-treated and untreated patients with the Zollinger-Ellison syndrome. Digestion. 1986; 35(Suppl 1):116–122.

25. Rindi G, Luinetti O, Cornaggia M, Capella C, Solcia E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology. 1993; 104:994–1006.

26. Cunningham R, Dale B, Undy B, Gaunt N. Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea. J Hosp Infect. 2003; 54:243–245.

27. Levine A, Shevah O, Shabat-Sehayek V, et al. Masking of 13C urea breath test by proton pump inhibitors is dependent on type of medication: comparison between omeprazole, pantoprazole, lansoprazole and esomeprazole. Aliment Pharmacol Ther. 2004; 20:117–122.

28. Iwahi T, Satoh H, Nakao M, et al. Lansoprazole, a novel benzimidazole proton pump inhibitor, and its related compounds have selective activity against Helicobacter pylori. Antimicrob Agents Chemother. 1991; 35:490–496.
29. Chey WD, Woods M, Scheiman JM, Nostrant TT, DelValle J. Lansoprazole and ranitidine affect the accuracy of the 14C-urea breath test by a pH-dependent mechanism. Am J Gastroenterol. 1997; 92:446–450.
30. Bugnoli M, Bayeli PF, Rappuoli O, et al. Inhibition of Helicobacter pylori urease by omeprazole. Eur J Gastroenterol Hepatol. 1993; 5:683–685.
31. Mauch F, Bode G, Malfertheiner P. Identification and characterization of an ATPase system of Helicobacter pylori and the effect of proton pump inhibitors. Am J Gastroenterol. 1993; 88:1801–1802.
32. Welage LS. Pharmacologic properties of proton pump inhibitors. Pharmacotherapy. 2003; 23:74S–80S.

33. Weil J, Bell GD, Powell K, et al. Omeprazole and Helicobacter pylori: temporary suppression rather than true eradication. Aliment Pharmacol Ther. 1991; 5:309–313.
Fig. 1.
Study design and flow. At 2 and 4 weeks, the false negative rates of 13 C-urea breath test (UBT) were 5.8% and 23.1%, respectively. After 2 weeks of the cessation of taking revaprazan, there were five patients of negative results. Among them, four patients had prolonged negative results on two or three successive tests.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download