Abstract
Background/Aims
Histologically confirmed metastatic pancreatic cancers are infrequent. The aim of this study was to analyze clinical, therapeutic and prognostic features of pancreatic metastases.
Methods
We retrospectively evaluated stage of primary malignancies, interval between diagnosis of primary tumors and detection of pancreatic metastases, treatment for metastases to the pancreas, survival rate, and prognostic factors in 31 patients with pancreatic metastases.
Results
The mean age at the time of primary cancer diagnosis was 52.4±13.2 years. Primary cancers were renal cell carcinoma (n=16), non-small cell lung cancer (n=6), small cell lung cancer (n=3), colorectal carcinoma (n=2), osteosarcoma (n=1), gastric carcinoma (n=1), malignant melanoma (n=1), and thymic carcinoma (n=1). Pancreatic metastases were synchronous in six cases and metachronous in twenty five cases, with median interval time of 40.8 months (range 3-186) between the diagnosis of primary tumor and detection of pancreatic metastases. The median survival after the detection of the metastases was 16 months. In multivariate analysis, non-renal cell carcinoma as primary malignancy and positive symptom related to pancreatic metastases were associated with poor prognosis (hazard ratio [HR], 8.33; 95% CI, 2.1-33; p=0.003, and HR, 4.02; 95% CI, 1.27-12.7; p=0.018).
Conclusions
Metastatic tumors to the pancreas have to be kept in mind when a patient with pancreatic mass has a history of other malignancy, even if treated several years before. In the absence of widely metastatic disease, aggressive diagnostic and therapeutic approach may offer the chance of long-term survival in selected patients.
Go to : 
References
1. Adsay NV, Andea A, Basturk O, Kilinc N, Nassar H, Cheng JD. Secondary tumors of the pancreas: an analysis of a surgical and autopsy database and review of the literature. Virchows Arch. 2004; 444:527–535.

2. Roland CF, van Heerden JA. Nonpancreatic primary tumors with metastasis to the pancreas. Surg Gynecol Obstet. 1989; 168:345–347.
3. Sperti C, Pasquali C, Liessi G, Pinciroli L, Decet G, Pedrazzoli S. Pancreatic resection for metastatic tumors to the pancreas. J Surg Oncol. 2003; 83:161–166.

4. Fabbri C, Luigiano C, Collina G, Cennamo V, D'Imperio N, Jovine E. EUS-FNA diagnosis of single pancreatic metastasis of liposarcoma. Gastrointest Endosc. 2009; 69:974–976.

5. Byun BH, Kang JH, Kim JA, Kim HK, Lee KS, Chang ED. Extraskel-etal mesenchymal chondrosarcoma of thigh with metastasis to pancreas: a case report and literature review. J Korean Cancer Assoc. 1995; 27:1070–1076.
6. Cho YD, Cho JY, Lee MS, et al. A case report of extrahepatic biliary obstruction caused by metastatic pancreatic small cell cancer. Korean J Gastroenterol. 1990; 22:1019–1026.
7. Choi CW, Lee HS, Lee BJ, et al. Lymphogenous pancreatic metastasis of gastric cancer detected by elevated CA 19-9 level. Korean J Gastrointest Endosc. 2005; 31:68–72.
8. Choi EK, Ju YM, Cha JM, et al. A case of metastatic hemangioper-icytoma of the pancreas. Korean J Gastroenterol. 2000; 35:534–538.
9. Koh KW, Kim HT, Jang SE, et al. A case of non-small cell lung cancer presenting as abdominal pain and a pancreatic nodule. Tuberc Respir Dis. 2009; 67:42–46.

10. Lee SH, Lee SK, Lee KH, et al. Renal cell carcinoma metastatic to the pancreas similar findings with pancreatic cancer in radiology. Korean J Med. 2006; 70(Suppl 2):S190–S193.
11. Lee TH, Chung IK, Gil HW, et al. Metastatic small cell carcinoma of the pancreas originating from the lung. Korean J Gastroenterol. 2003; 41:417–420.
12. Yoon WJ, Park JK, Lee SH, et al. Metastatic tumors of the pancreas. Korean J Med. 2007; 72:266–271.
13. Cubilla AL, Fitzgerald PJ. Cancer of the exocrine pancreas: the pathologic aspects. CA Cancer J Clin. 1985; 35:2–18.

14. Nakamura E, Shimizu M, Itoh T, Manabe T. Secondary tumors of the pancreas: clinicopathological study of 103 autopsy cases of Japanese patients. Pathol Int. 2001; 51:686–690.

15. Hiotis SP, Klimstra DS, Conlon KC, Brennan MF. Results after pancreatic resection for metastatic lesions. Ann Surg Oncol. 2002; 9:675–679.

16. Klein KA, Stephens DH, Welch TJ. CT characteristics of metastatic disease of the pancreas. Radiographics. 1998; 18:369–378.

17. Minni F, Casadei R, Perenze B, et al. Pancreatic metastases: ob-servations of three cases and review of the literature. Pancreatology. 2004; 4:509–520.

18. Kassabian A, Stein J, Jabbour N, et al. Renal cell carcinoma metastatic to the pancreas: a single-institution series and review of the literature. Urology. 2000; 56:211–215.

19. Sandock DS, Seftel AD, Resnick MI. A new protocol for the follow-up of renal cell carcinoma based on pathological stage. J Urol. 1995; 154:28–31.

20. Z'graggen K, Fernández-del Castillo C, Rattner DW, Sigala H, Warshaw AL. Metastases to the pancreas and their surgical extirpation. Arch Surg. 1998; 133:413–417.
21. McNichols DW, Segura JW, DeWeerd JH. Renal cell carcinoma: long-term survival and late recurrence. J Urol. 1981; 126:17–23.

22. Wente MN, Kleeff J, Esposito I, et al. Renal cancer cell metastasis into the pancreas: a single-center experience and over-view of the literature. Pancreas. 2005; 30:218–222.
Go to : 
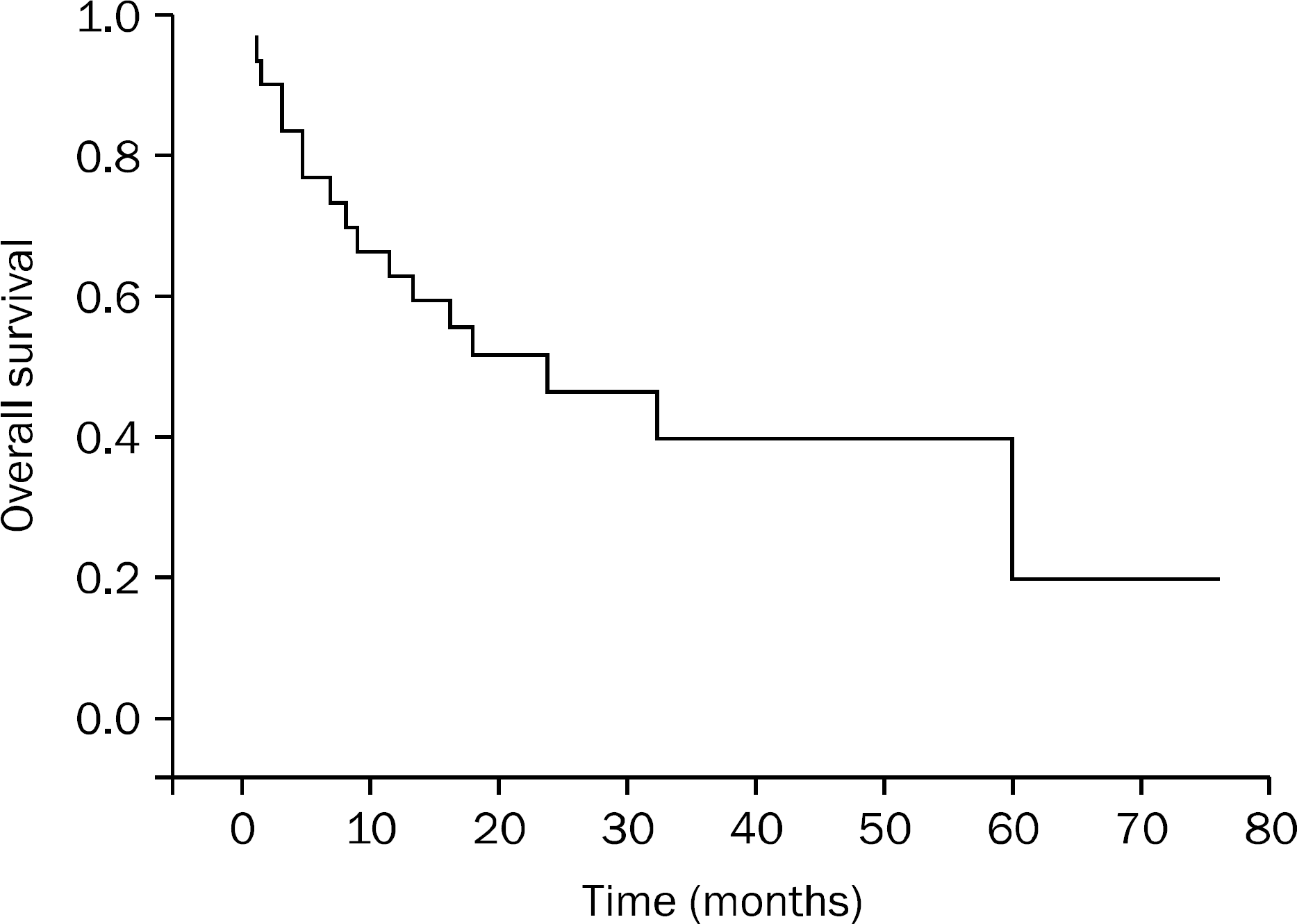 | Fig. 1.Kaplan-Meier survival curve after detection of pancreatic metastases. The median survival for patients with pancreatic metastases was 16 months. |
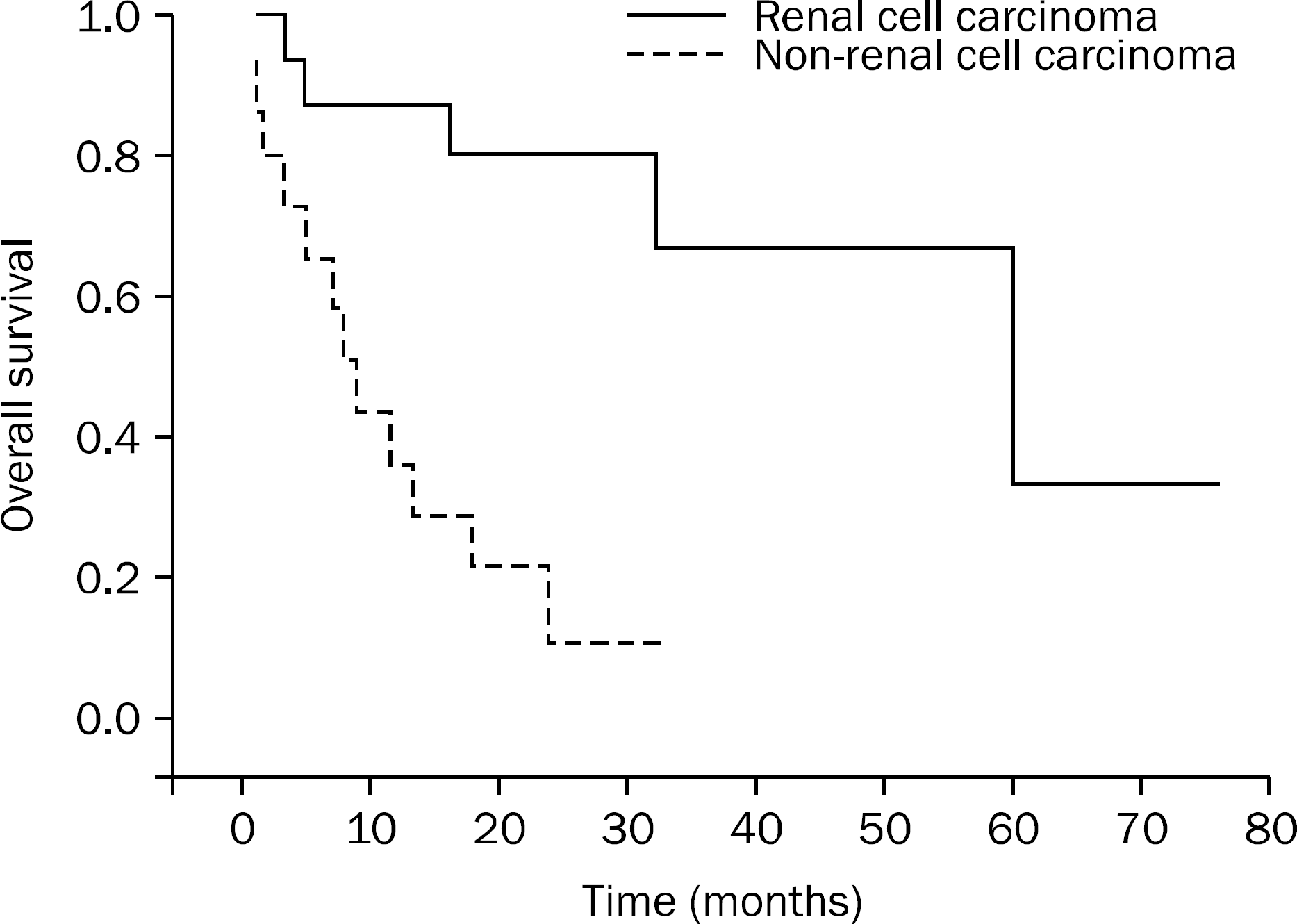 | Fig. 2.Survival comparison between patients with pancreatic renal cell carcinoma metastases and patients with pancreatic non-renal cell carcinoma metastases. Renal cell carcinoma as primary cancer was associated with prolonged survival (mean 52 vs. 12 months, p=0.001). |
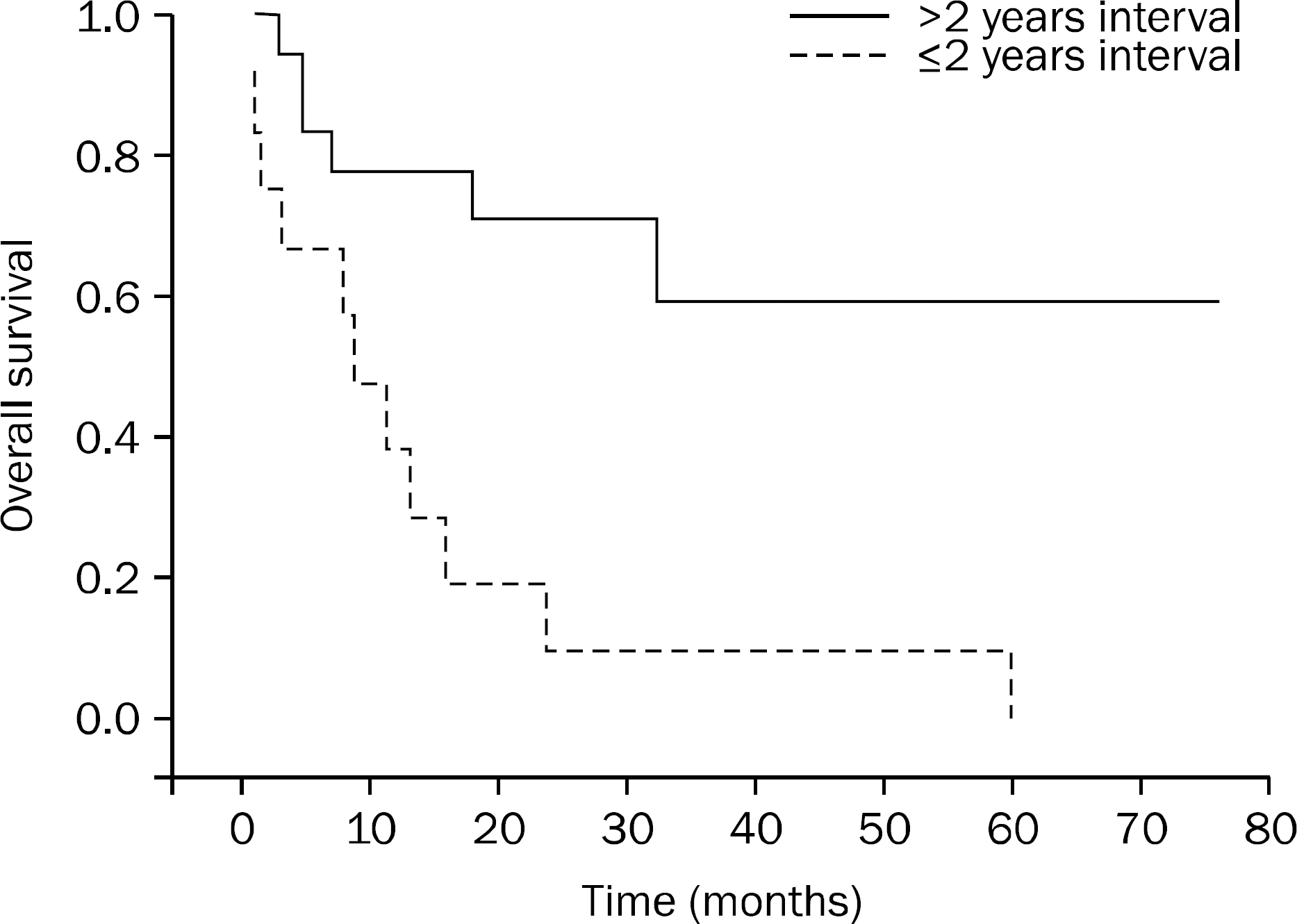 | Fig. 3.Survival comparison between ≤2 years interval and >2 years interval between diagnosis of primary tumors and detection of pancreatic metastases. The interval of more than 2 years was associated with prolonged survival (mean 51 vs. 14 months, p=0.001). |
Table 1.
Clinical Findings of 31 Patients with Metastases to the Pancreas
Table 2.
Univariate Analysis for Potential Predictors of Overall Survival after Detection of Pancreatic Metastases
Table 3.
Multivariate Analysis for Potential Predictors of Overall Survival after Detection of Pancreatic Metastases




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download