Abstract
Background/Aims
P2/MS is a noninvasive marker for detecting hepatic fibrosis in patients with viral hepatitis. However, the applicability of P2/MS in patients with nonalcoholic fatty liver disease (NAFLD) has not yet been validated. This study aimed to validate P2/MS and compare it to other noninvasive fibrosis scoring systems in Korean patients with NAFLD.
Methods
Consecutive patients who underwent liver biopsy between January 2002 and December 2009 at Seoul National University Hospital, Seoul, Korea were enrolled in this study. Fibrosis stage was determined using the METAVIR scoring system.
Results
A total of 235 patients were included in the study: advanced fibrosis (METAVIR F3-F4) was present in 7 patients. No patient was over-staged among 162 patients with a P2/MS score above the high cut-off (95), resulting in a high negative predictive value (NPV) of 100% (95% confidence interval, 97.1–100). There was no significant difference between the area under the receiver-operating characteristic curve (AUROC) of the FIB-4 (0.964) and the AUROC of the NAFLD fibrosis score (0.964) or P2/MS (0.940) for detecting advanced fibrosis. If P2/MS was implemented in the Korean patients with NAFLD, 68.9% of liver biopsies might be avoided.
Conclusions
P2/MS has a high NPV for excluding advanced fibrosis in Korean patients with NAFLD, and can reduce the burden of liver biopsy in the majority of cases. Since there were few patients with advanced fibrosis, further studies are warranted in a cohort including more patients with advanced fibrosis to validate the low cut-off value.
Go to : 
References
1. Wong VW, Wong GL, Chim AM, et al. Validation of the NAFLD fibrosis score in a Chinese population with low prevalence of advanced fibrosis. Am J Gastroenterol. 2008; 103:1682–1688.

2. Targher G, Bertolini L, Padovani R, Zenari L, Zoppini G, Falezza G. Relation of nonalcoholic hepatic steatosis to early carotid atherosclerosis in healthy men: role of visceral fat accumulation. Diabetes Care. 2004; 27:2498–2500.
4. Guha IN, Parkes J, Roderick P, et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology. 2008; 47:455–460.

5. Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010; 51:1820–1832.

6. Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005; 42:132–138.

7. Ekstedt M, Franzén LE, Mathiesen UL, et al. Longterm follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006; 44:865–873.

8. Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007; 45:846–854.

9. Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003; 38:1449–1457.

10. Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005; 128:1898–1906.

11. Grattagliano I, D'Ambrosio G, Palmieri VO, Moschetta A, Palasciano G, Portincasa P. "Steatostop Project" Group. Improving nonalcoholic fatty liver disease management by general practitioners: a critical evaluation and impact of an educational training program. J Gastrointestin Liver Dis. 2008; 17:389–394.
12. McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010; 59:1265–1269.

13. Williams AL, Hoofnagle JH. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology. 1988; 95:734–739.

14. Bourliere M, Penaranda G, Renou C, et al. Validation and comparison of indexes for fibrosis and cirrhosis prediction in chronic hepatitis C patients: proposal for a pragmatic approach classification without liver biopsies. J Viral Hepat. 2006; 13:659–670.

15. Borroni G, Ceriani R, Cazzaniga M, et al. Comparison of simple tests for the non-invasive diagnosis of clinically silent cirrhosis in chronic hepatitis C. Aliment Pharmacol Ther. 2006; 24:797–804.

16. Blonsky JJ, Harrison SA. Review article: nonalcoholic fatty liver disease and hepatitis C virus–partners in crime. Aliment Pharmacol Ther. 2008; 27:855–865.
17. Islam S, Antonsson L, Westin J, Lagging M. Cirrhosis in hepatitis C virus-infected patients can be excluded using an index of standard biochemical serum markers. Scand J Gastroenterol. 2005; 40:867–872.

18. Bonacini M, Hadi G, Govindarajan S, Lindsay KL. Utility of a discriminant score for diagnosing advanced fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1997; 92:1302–1304.
19. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006; 43:1317–1325.

20. Wong VW, Hui AY, Tsang SW, et al. Metabolic and adi-pokine profile of Chinese patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2006; 4:1154–1161.

21. Lee JH, Yoon JH, Lee CH, et al. Complete blood count reflects the degree of oesophageal varices and liver fibrosis in virus-related chronic liver disease patients. J Viral Hepat. 2009; 16:444–452.

22. Kim BK, Han KH, Park JY, et al. External validation of P2/MS and comparison with other simple non-invasive indices for predicting liver fibrosis in HBV-infected patients. Dig Dis Sci. 2010; 55:2636–2643.

23. Kim BK, Han KH, Park JY, et al. Prospective validation of P2/MS noninvasive index using complete blood counts for detecting oesophageal varices in B-viral cirrhosis. Liver Int. 2010; 30:860–866.

24. Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005; 42:641–649.

25. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996; 24:289–293.
26. Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for non-alcoholic fatty liver disease. Hepatology. 2005; 41:1313–1321.

27. Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983; 148:839–843.

28. Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ. Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with non-alcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009; 7:1104–1112.

29. Matsumura H, Moriyama M, Goto I, Tanaka N, Okubo H, Arakawa Y. Natural course of progression of liver fibrosis in Japanese patients with chronic liver disease type Ca study of 527 patients at one establishment. J Viral Hepat. 2000; 7:268–275.
30. Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003; 37:1286–1292.

31. Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007; 46:32–36.

32. Malik A, Cheah PL, Hilmi IN, Chan SP, Goh KL. Non-alcoholic fatty liver disease in Malaysia: a demographic, anthropometric, metabolic and histological study. J Dig Dis. 2007; 8:58–64.

Go to : 
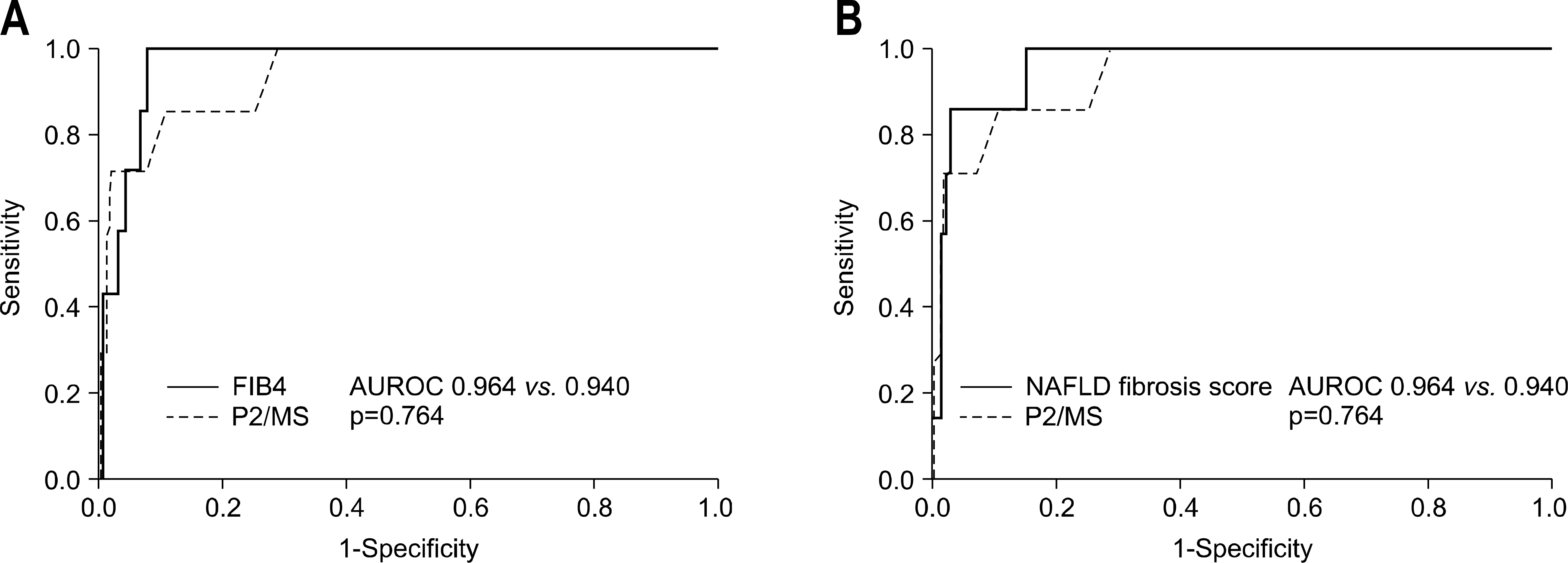 | Fig. 1.Comparisons of the ROC of P2/MS and other noninvasive fibrosis scoring systems for the diagnosis of advanced fibrosis (defined as METAVIR F3-F4). (A) P2/MS versus FIB-4 and (B) P2/MS versus NAFLD fibrosis score. |
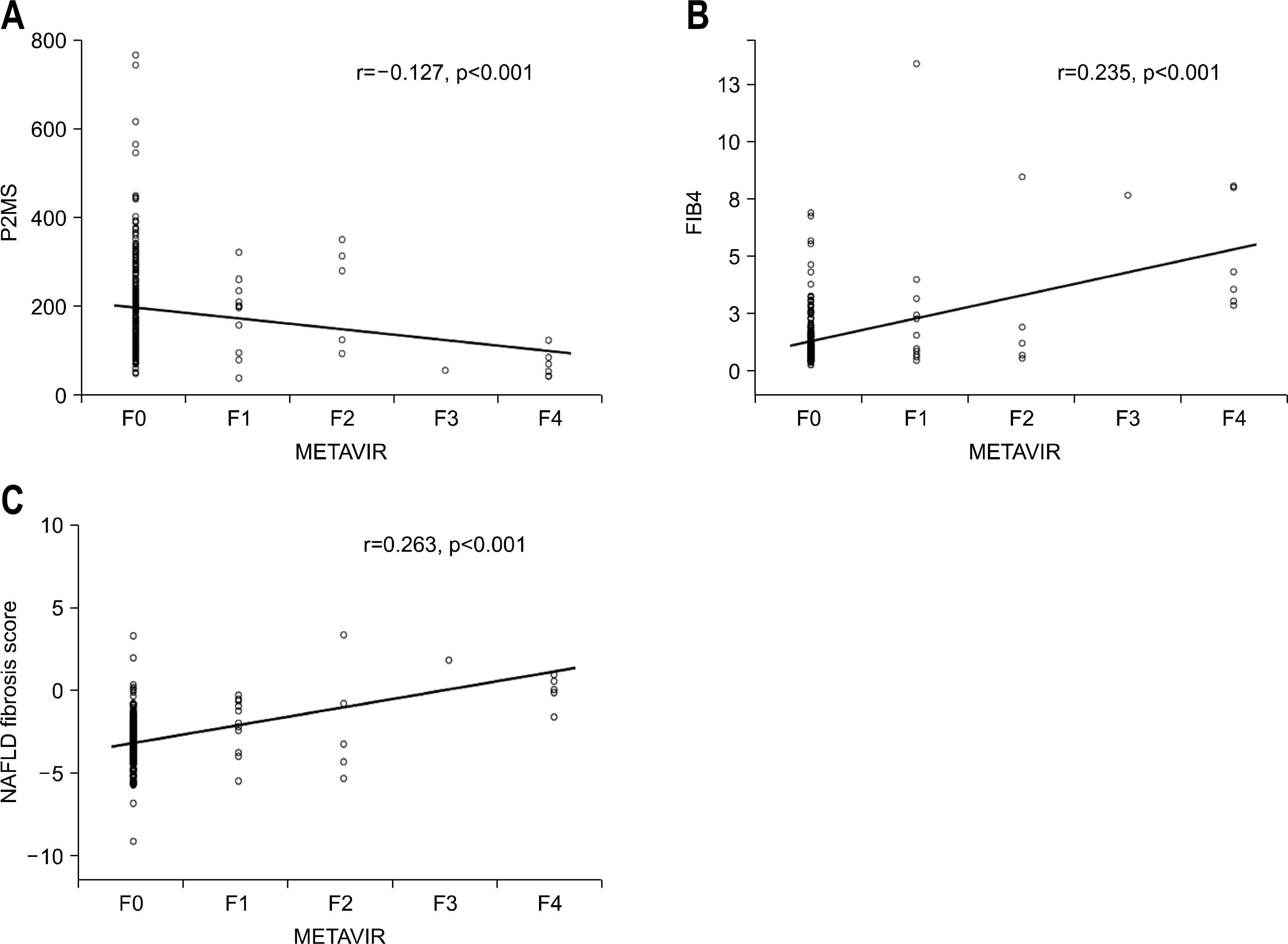 | Fig. 2.Scatter plot of noninvasive fibrosis scoring systems versus pathologically determined fibrosis stage. Fibrosis stage was determined using the METAVIR scoring system.25 Circles represent the values of each noninvasive fibrosis scoring systems. The solid line represents a trend-line. (A) P2/MS values showed a significant inverse correlation with the METAVIR fibrosis stage (Spearman's correlation coefficient=−0.127, p<0.001), (B) FIB-4 scores showed a significant inverse correlation with the METAVIR fibrosis stage (Spearman's correlation coefficient=0.235, p<0.001), (C) NAFLD fibrosis score values showed a significant inverse correlation with the METAVIR fibrosis stage (Spearman's correlation coefficient= 0.263, p<0.001). |
Table 1.
Formulae of Noninvasive Fibrosis Scoring Systems
Table 2.
Baseline Characteristics of All Patients
| Variables | F0-F2 (n=228) | F3-F4 (n=7) | p-value |
|---|---|---|---|
| Age (years) | 40.6±8.1 | 60.4±15.3 | 0.001 a |
| Male, n (%) | 124 (54.4) | 1 (14.3) | 0.037 b |
| BMI (kg/m2) | 24.1±3.1 | 30.2±6.3 | 0.004 a |
| Waist circumference (cm) | 81.7±7.7 | 90.7±11.0 | 0.026 a |
| Diabetes, n (%) | 30 (13.2) | 1 (14.3) | 1.000 b |
| IFG, n (%) | 57 (25) | 2 (28.6) | 1.000 b |
| Platelet count (109/L) | 238.4±71.1 | 115.0±38.9 | <0.001 a |
| Total bilirubin (mg/dL) | 1.5±2.8 | 1.2±0.4 | 0.337 a |
| Albumin (g/dL) | 3.9±0.6 | 3.4±0.7 | 0.071 a |
| AST (IU/L) | 53.5±57.5 | 60.0±35.0 | 0.167 a |
| ALT (IU/L) | 66.0±77.9 | 46.3±32.3 | 0.944 a |
| Prothrombin time (INR) | 1.02±0.07 | 1.25±0.10 | <0.001 a |
| Fasting glucose (mg/dL) | 110.6±32.0 | 120.7±50.8 | 0.622 a |
| HbA1c (%) | 5.57±0.53 | 6.95±1.91 | 0.170 a |
| Total cholesterol (mg/dL) | 159.4±45.8 | 142.0±35.1 | 0.336 a |
| HDL-cholesterol | 50.3±13.1 | 40.7±6.47 | 0.049 a |
| LDL-cholesterol | 85.2±42.0 | 64.4±54.3 | 0.211 a |
| Triglycerides | 133.1±274.0 | 181.5±100.5 | 0.020 a |
Table 3.
AUROC of Noninvasive Fibrosis Scoring Systems for Predicting Advanced Fibrosis (METAVIR F3-F4)
| Noninvasive fibrosis scoring systems | AUROC (95% CI) | p-value a |
|---|---|---|
| P2/MS | 0.940 (0.841–1.000) | − |
| FIB-4 | 0.964 (0.935–0.992) | 0.764 |
| NAFLD fibrosis score | 0.964 (0.917–1.000) | 0.764 |
| Cirrhosis discriminant score: Score is the sum of three (0–11) | 0.936 (0.859–1.000) | 0.964 |
| Goteborg University Cirrhosis Index | 0.860 (0.780–0.940) | 0.465 |
| AST to platelet ratio index | 0.825 (0.737–0.914) | 0.320 |
| AST to platelet ratio | 0.825 (0.737–0.914) | 0.320 |
| AST to ALT ratio | 0.787 (0.687–0.888) | 0.206 |
| BARD score: Scale 0–4 | 0.691 (0.542–0.841) | 0.054 |
| Simple panel for distinguishing moderate fibrosis | 0.691 (0.464–0.919) | 0.054 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download