Abstract
Background/Aims
Acute hepatitis A (HAV) is markedly increasing recently on. Some patients with acute hepatitis A show severe clinical course. The seroprevalence rate of IgG anti-HAV has been changing with the regions and the times. Vaccination and seroconversion rate of HAV are not well known. In this study, we aimed to study the difference of seroprevalence rate of IgG anti-HAV according to various clinical factors and to know the vaccination rate and seroconversion rate below 10 years old in the central region of South Korea including Cheonan city.
Methods
Seven hundred seventy two subjects were included in the study from January to September 2009. We analyzed seroprevalence rate of IgG anti-HAV according to sex, age, region, and other viral markers. We interviewed the history of vaccination(1st, 2nd) and analyzed seroconversion rate according to vaccination time below 10 years old.
Go to : 
References
1. Feinstone SM, Kapikian AZ, Purceli RH. Hepatitis A: detection by immune electron microscopy of a viruslike antigen associated with acute illness. Science. 1973; 182:1026–1028.

2. Hadler SC, Webster HM, Erben JJ, Swanson JE, Maynard JE. Hepatitis A in day-care centers. A community-wide assessment. N Engl J Med. 1980; 302:1222–1227.
3. Lee CH, Chung KW, Moon YM, Yoo JY, Sub DJ, Lee SG. An outbreak of hepatitis A in Korean young adults in 1998 [abstract]. Korean J Gastroenterol. 1998; 32(Suppl 1):105A.
4. André F, Van Damme P, Safary A, Banatvala J. Inactivated hepatitis A vaccine: immunogenicity, efficacy, safety and review of official recommendations for use. Expert Rev Vaccines. 2002; 1:9–23.

5. Jilg W, Bittner R, Bock HL, et al. Vaccination against hepatitis A: comparison of different short-term immunization schedules. Vaccine. 1992; 10(Suppl 1):S126–S128.

6. Van Damme P, Matheï C, Thoelen S, Meheus A, Safary A, André FE. Single dose inactivated hepatitis A vaccine: rationale and clinical assessment of the safety and immunogenicity. J Med Virol. 1994; 44:435–441.

7. DeFraites RF, Feighner BH, Binn LN, et al. Immunization of US sol-diers with a two-dose primary series of inactivated hepatitis A vaccine: early immune response, persistence of antibody, and response to a third dose at 1 year. J Infect Dis. 1995; 171(Suppl 1):S61–S69.

8. Müller R, Chriske H, Deinhardt F, et al. Hepatitis A vaccination: schedule for accelerated immunization. Vaccine. 1992; 10(Suppl 1):S124–S125.

9. Just M, Berger R. Reactogenicity and immunogenicity of inactivated hepatitis A vaccines. Vaccine. 1992; 10(Suppl 1):S110–S113.

10. Victor J, Knudsen JD, Nielsen LP, et al. Hepatitis A vaccine. A new convenient single-dose schedule with booster when long-term immunization is warranted. Vaccine. 1994; 12:1327–1329.

11. Hadler SC. Global impact of hepatitis A virus infection; changing patterns. Hollinger FB, Lemon SM, Margolis HS, editors. Viral hepatitis and liver diseases. Baltimore: Williams & Wilkins;1991. p. 14–20.
13. Weitz M, Siegl G. Variation among hepatitis A virus strains. I. Genomic variation detected by T1 oligonucleotide mapping. Virus Res. 1985; 4:53–67.

14. Sjogren MH. Hepatitis A. Feldman M, Friledman LS, Brandt LJ, editors. Sleigenger & Fordtran's gastrointestinal and liver disease. Vol 2. 8th ed.Philadelphia: Saunders;2006. p. 1639–1646.

15. Shapiro CN, Coleman PJ, McQuillan GM, Alter MJ, Margolis HS. Epidemiology of hepatitis A: seroepidemiology and risk groups in the USA. Vaccine. 1992; 10(Suppl 1):S59–S62.

16. Bell PB. Global epidemiology of hepatitis A: Implications for control strategies. 10th International Symposium on Viral Hepatitis and Liver Disease. 2000 Apr. 9–13;Atlanta, USA.2002.
17. Nelson KE. Global changes in the epidemiology of hepatitis A virus infections. Clin Infect Dis. 2006; 42:1151–1152.

18. Hong WS, Kim CY. Seroepidemiology of type A and type B hepatitis in Seoul area. Korean J Intern Med. 1982; 25:19–26.
19. Kim TW, Lee KJ. Antibody to hepatitis A antigen in children and adolescent in Korea. J Korean Pediatr Soc. 1982; 25:36–40.
20. Lim DS, Cho KH, Kim HC. Seroepidemiological study on anti-HAV antibody in Cheon-Buk Province in 1989. Korean J Intern Med. 1992; 43:57–65.
21. Sohn YM, Rho HO, Park MS, et al. The changing epidemiology of hepatitis A in children and the consideration of active immunization in Korea. Yonsei Med J. 2000; 41:34–39.

22. Yang DW, Lee YA, Shim JY, et al. A seroepidemiologic study on hepatitis A in Seoul, Korea. J Korean Pediatr Soc. 1999; 42:180–185.
23. Song YB, Lee JH, Choi MS, et al. The age-specific seroprevalence of hepatitis A virus antibody in Korea. Korean J Hepatol. 2007; 13:27–33.
24. Pramoolsinsap C. Acute hepatitis A and acquired immunity to hepatitis A virus in hepatitis B virus (HBV) carriers and in HBV- or hepatitis C virus-related chronic liver diseases in Thailand. J Viral Hepat. 2000; 7(Suppl 1):11–12.

25. Chu CM, Liaw YF. Increased incidence of fulminant hepatic failure in previously unrecognized HBsAg carriers with acute hepatitis independent of etiology. Infection. 2005; 33:136–139.

26. Sohn YM, Rho HO, Park MS, et al. The changing epidemiology of hepatitisA in children and the consideration of active immunization in Korea. Yonsei Med J. 2000; 41:34–39.
27. Kim JH. Recent epidemiological status and vaccination of hepatitis A in Korea. J Korean Med Assoc. 2008; 51:110–118.

28. Iwarson S. Are we giving too many doses of hepatitis A and B vac-cines? Vaccine. 2002; 20:2017–2018.

29. Williams J, Bruden D, McMahon B, et al. Response to two doses of hepatitis A vaccine administered an average of 27 months apart. Antiviral Ther. 2000; 13:5.
30. Landry P, Tremblay S, Darioli R, Genton B. Inactivated hepatitis A vaccine booster given at least 24 months after the primary dose. Vaccine. 2000; 19:399–402.
Go to : 
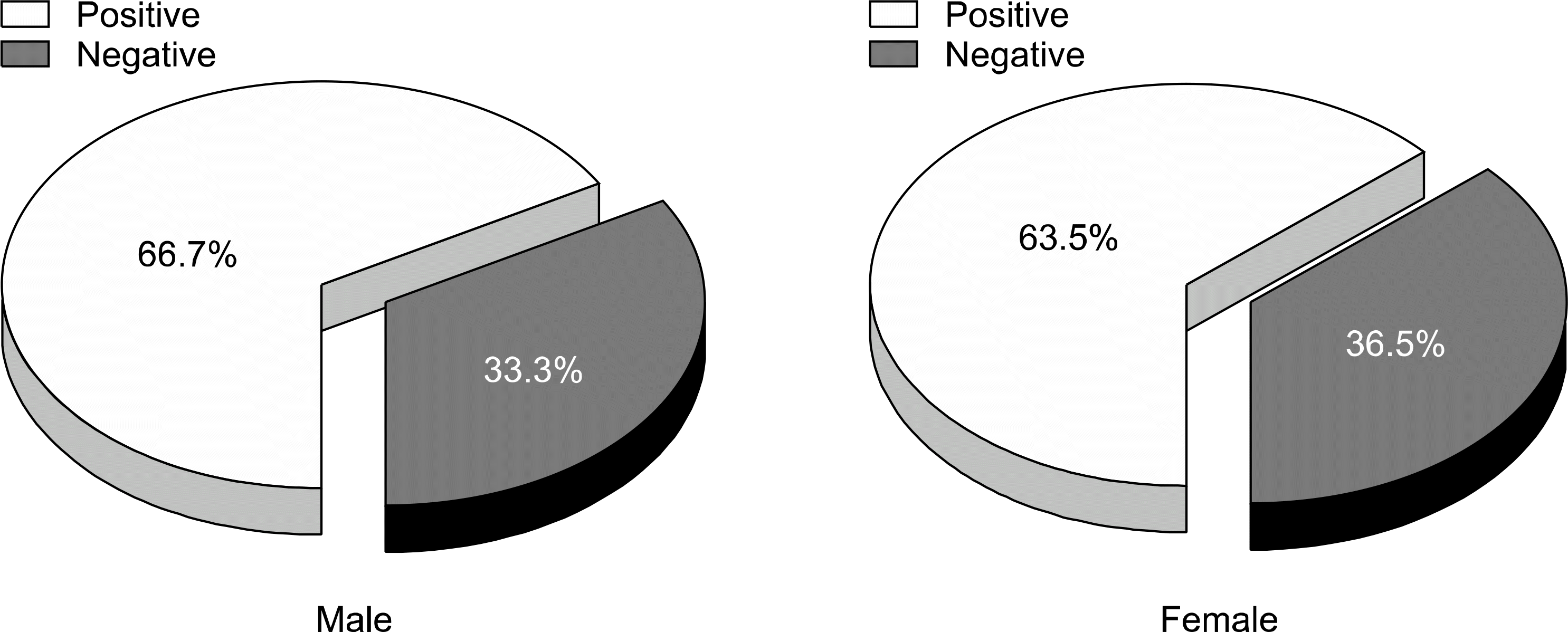 | Fig. 1.The seroprevalence rate of IgG anti-HAV according to sex. There was no statistical difference (p>0.05). |
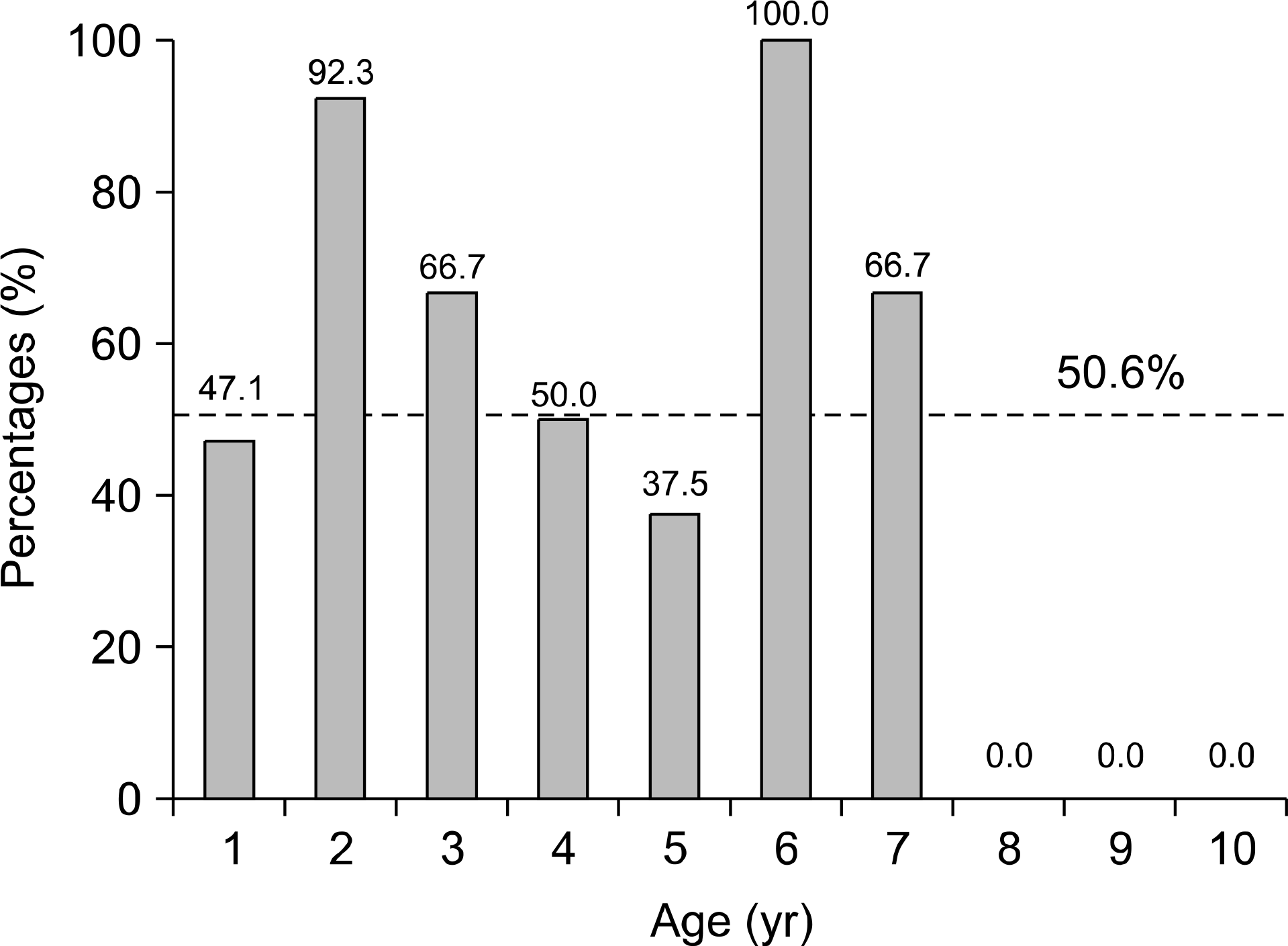
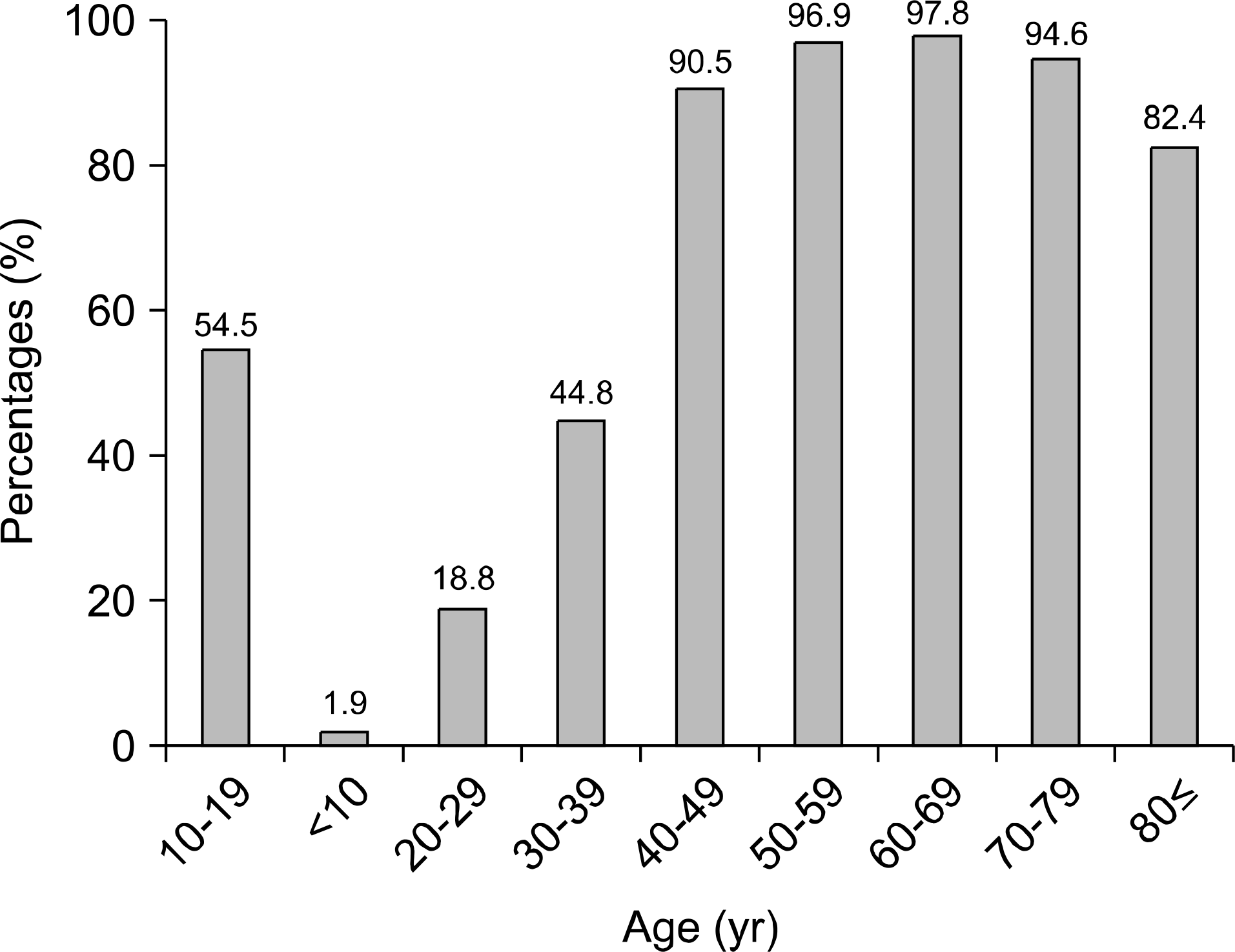 | Fig. 2.The seroprevalence rate of IgG anti-HAV according to age group. The seroprevalence rate was lowest in second and third decade. |
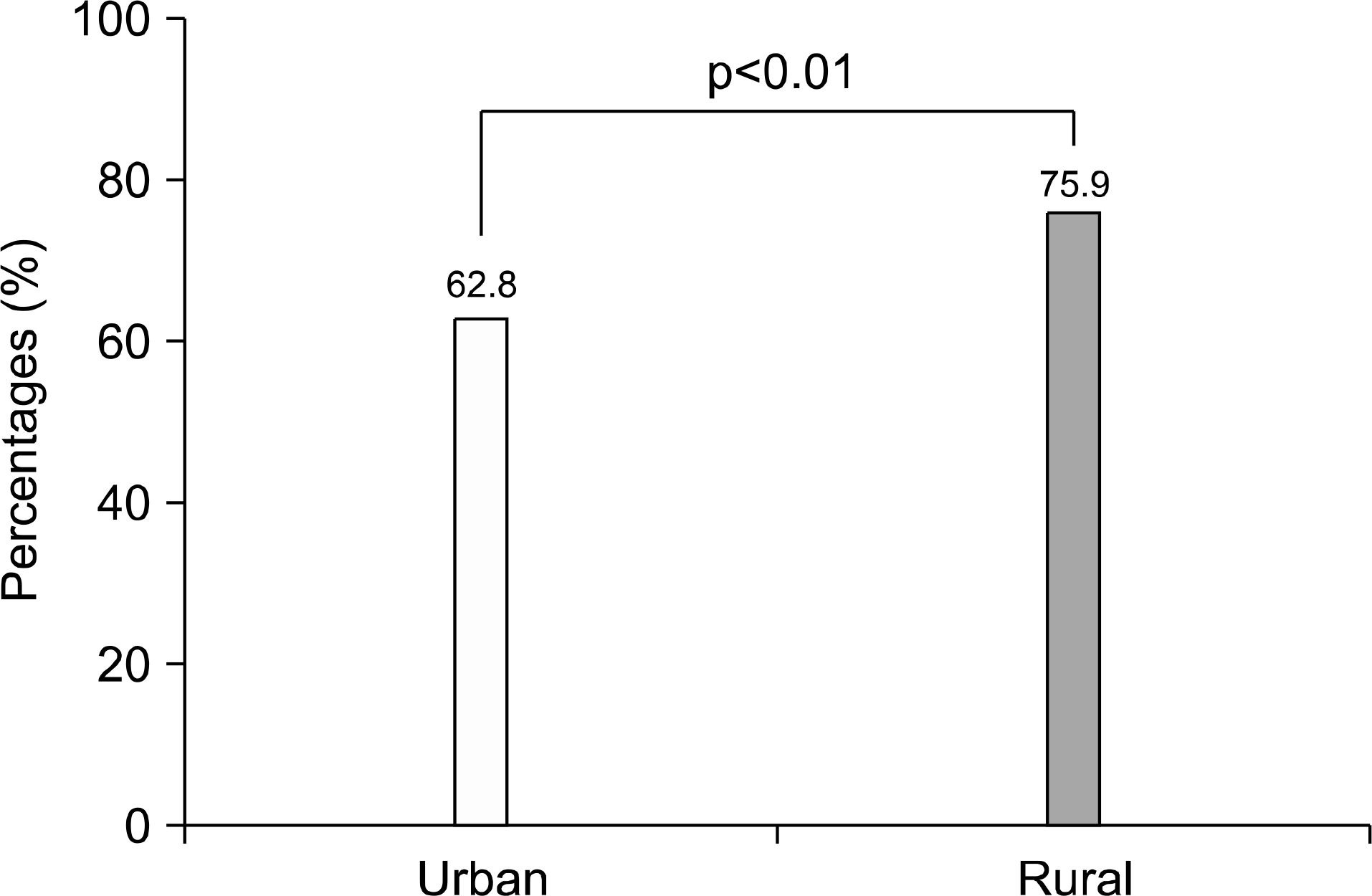 | Fig. 3.The seroprevalence rate of IgG anti-HAV according to region. The seroprevalence rate of rural was higher than that of urban (p <0.01). |
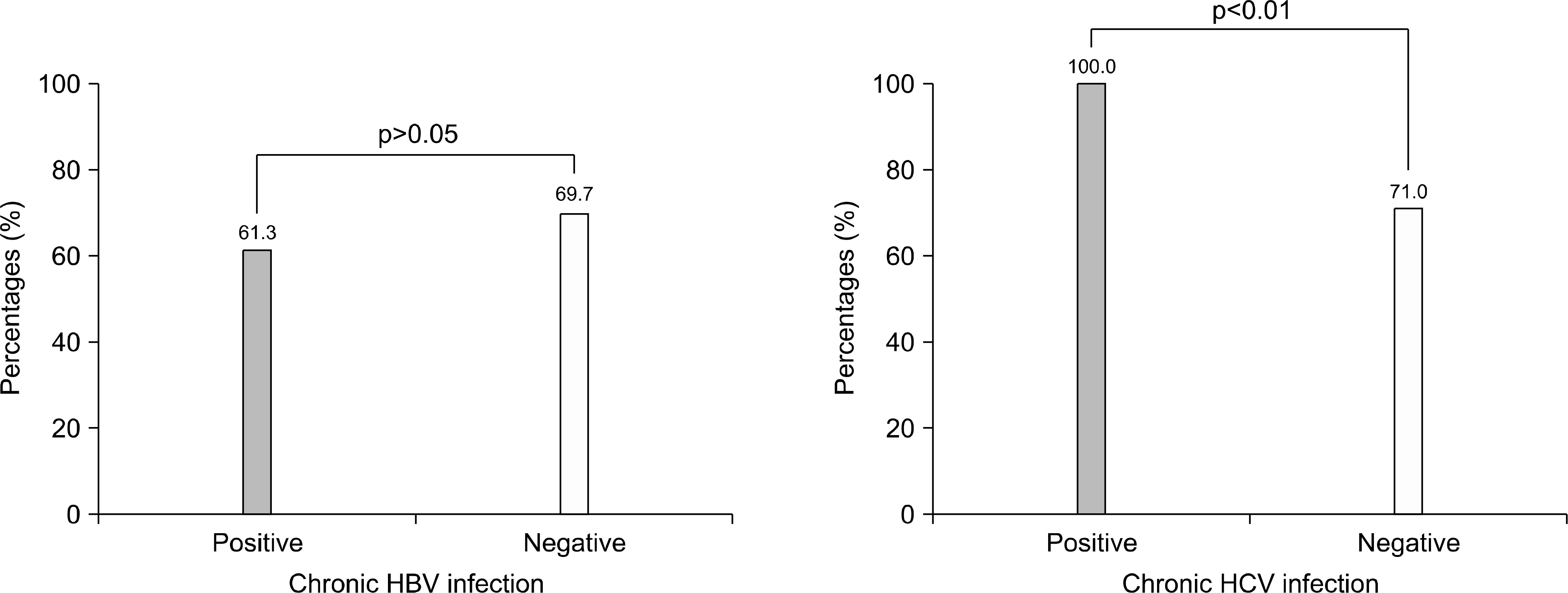 | Fig. 4.The seroprevalence rate of IgG anti-HAV according to viral markers. Patients with chronic hepatitis B did not show difference in seroprevalence rate of IgG anti HAV (p>0.05). Patients with chronic hepatitis C showed higher seroprevalence rate of IgG anti-HAV (p<0.01). |
Table 1.
Subjects Distribution according to Sex and Age




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download