Abstract
Background/Aims
Pyogenic liver abscess remains a major diagnostic and therapeutic challenge, despite advances in diagnostic technology and new strategies for treatment. This study was conducted to compare the differences in clinical features and outcomes of pyogenic liver abscess according to age.
Methods
In total, 166 patients were enrolled and included 63 (<65 years old, group I), 62 (65-74 years old, group II), 41 (>75 years old, group III) patients in each group. We reviewed the medical records retrospectively including etiology, underlying diseases, characteristics of the liver abscess, laboratory and microbiologic findings, treatment, and outcome of the patients.
Results
Group I had higher prevalence rates of male patients and chronic alcoholics, but lower prevalence rates of biliary disease, hypertension, and malignancy. In laboratory findings, group II had higher incidence of thrombocytopenia, elevated blood urea nitrogen and creatinine. There were no differences in symptoms and microbiologic findings in blood and pus among the three groups. Liver abscesses were more common in right liver in Group I. The lengths of stay and the treatment modalities were similar in three groups.
REFERENCES
1. Alvarez JA, González JJ, Baldonedo RF, et al. Pyogenic liver abscesses: a comparison of older and younger patients. HPB(Oxford). 2001; 3:201–206.

2. Ha J, Choi SP, Lee WH, et al. A clinical study on pyogenic liver abscesses: the changes in the clinical features durting the recent 12 years. Korean J Med. 2008; 74:37–50.
3. Gerzof SG, Johnson WC, Robbins AH, Nabseth DC. Intrahepatic pyogenic abscesses: treatment by percutaneous drainage. Am J Surg. 1985; 149:487–494.
4. Kim YH, Park KS. A clinical study of liver abscess. J Kor Surg Soc. 1980; 22:157–167.
5. Seo TJ, Park CH, Lee SH, et al. A clinical study on liver abscess for recent 15 years in Gwanjuㆍ Chonnam province. Korean J Med. 2005; 68:26–39.
6. Chen SC, Lee YT, Yen CH, et al. Pyogenic liver abscess in the elderly: clinical features, outcomes and prognostic factors. Age Ageing. 2009; 38:271–276.

7. Choi HY, Cheon GJ, Kim YD, Han KH, Kim KS, Nah BK. Comparison of clinical characteristics between cryptogenic and biliary pyogenic liver abscess. Korean J Gastroenterol. 2007; 49:238–244.
8. Park BK, Chun JB, Hahm JS, et al. A clinical study on pyogenic liver abscesses. Korean J Gastroenterol. 1992; 24:1347–1361.
9. Nah BK, Kim YS, Moon HS, et al. Recent changes of organism and treatment in pyogenic liver abscess. Korean J Hepatol. 2003; 9:275–283.
10. Lee CJ, Jung DS, Jung SH, et al. Comparison of liver abscess between diabetic patients and non-diabetic patients. Korean J Hepatol. 2005; 11:339–349.
11. Rubin RH, Swartz MN, Malt R. Hepatic abscess: changes in clinical, bacteriologic and therapeutic aspects. Am J Med. 1974; 57:601–610.

12. Branum GD, Tyson GS, Branum MA, Meyes WC. Hepatic abscess. Change in etiology, diagnosis, and management. Ann Surg. 1990; 212:655–662.
13. Foo NP, Chen KT, Lin HJ, Guo HR. Characteristics of pyogenic liver abscess patients with and without diabetes mellitus. Am J Gastroenterol. 2010; 105:328–335.

14. Lim SW, Lee EJ, Lee SW, et al. Clinical significance of Klebsiella pneumoniae in liver abscesses. Korean J Gastroenterol. 2003; 42:226–231.
15. Cerwenka H. Pyogenic liver abscess: differences in etiology and treatment in Southeast Asia and Central Europe. World J Gastroenterol. 2010; 16:2458–2462.

16. Chesn SC, Wu WY, Yeh CH, et al. Comparison of Escherichia coli and Klebsiella pneumoniae liver abscesses. Am J Med Sci. 2007; 334:97–105.
17. Smoger SH, Mitchell CK, MaClave SA. Pyogenic liver abscesses: a comparison of older and younger patients. Age Ageing. 1998; 27:443–448.

18. Lee OJ, Kim YC. A clinical study on liver abscess. Korean J Gastroenterol. 1994; 26:506–520.
19. Kim IS, Lee BE, Jeon SY, Kim SW. Treatment of pyogenic abscess by percutaneous needle aspiration and/or percutaneous catheter drainage. Korean J Gastroenterol. 1999; 33:58–66.
20. Bertel CK, van Heerden JA, Sheedy PF 2nd. Treatment of pyogenic liver abscesses: surgical vs percutaneous drainage. Arch Surg. 1986; 121:554–558.
21. Lee KT, Sheen PC, Chen JS, Ker CG. Pyogenic liver abscess: multivariate analysis of risk factors. World J Surg. 1991; 15:372–376.

22. Chou FF, Sheen-Chen SM, Chen YS, Chen MC, Chen FC, Tai DI. Prognostic factors for pyogenic abscess of the liver. J Am Coll Surg. 1994; 179:727–732.
23. Pitt HA, Zuidema GD. Factors influencing mortality in the treatment of pyogenic hepatic abscess. Surg Gynecol Obstet. 1975; 140:228–234.
24. Yang CC, Yen CH, Ho MW, Wang JH. Comparison of pyogenic liver abscess caused by non-Klebsiella pneumoniae and Klebsiella pneumoniae. J Microbiol Immunol Infect. 2004; 37:176–184.
Fig. 1.
Presenting symptoms of pyogenic liver abscess. Other symptoms include flank pain, diarrhea, chest pain.
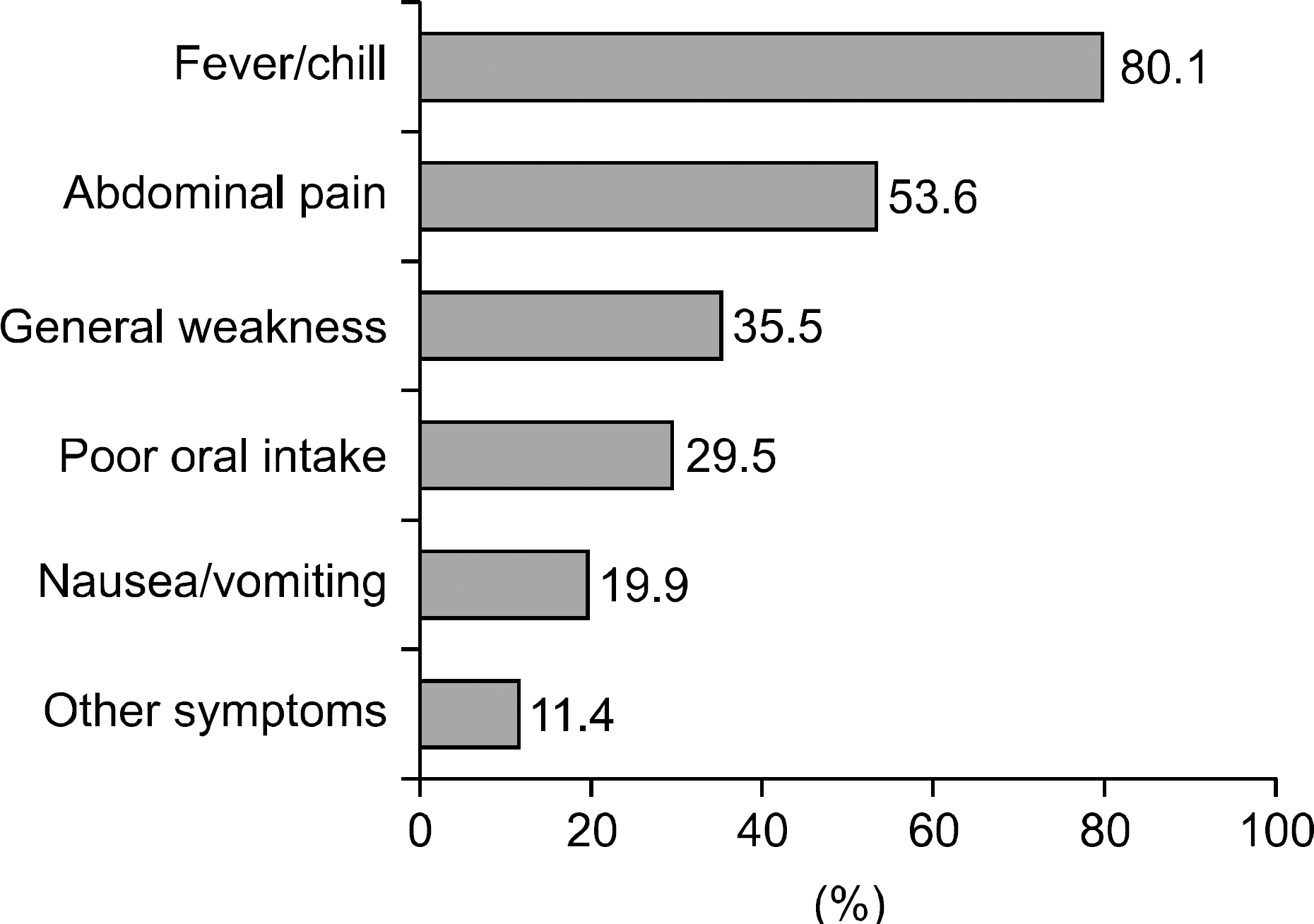
Table 1.
Comparison of Underlying Disease among Three Age Groups
| Total (n=166) | Age (years) | |||
|---|---|---|---|---|
| <65 (n=63) | 65-74 (n=62) | ≥75 (n=41) | ||
| Male | 98 (59.0%) | 45 (71.4%) | 37 (59.7%) | 16 (39.0%)∗ |
| Biliary disease | 53 (31.9%) | 9 (14.3%) | 25 (40.3%) | 19 (46.3%)∗ |
| Diabetes mellitus | 52 (31.3%) | 16 (25.4%) | 25 (40.3%) | 11 (26.8%) |
| Hypertension | 50 (30.1%) | 7 (11.1%) | 25 (40.3%) | 18 (43.9%)∗ |
| Liver cirrhosis | 12 (7.2%) | 6 (9.5%) | 4 (6.5%) | 2 (4.9%) |
| Chronic alcoholism | 44 (26.5%) | 26 (41.3%) | 14 (22.6%) | 4 (9.8%)∗ |
| Malignancy | 31 (18.7%) | 8 (12.7%) | 10 (16.1%) | 13 (31.7%)∗ |
Table 2.
Comparison of Initial Laboratory Data among Three Age Groups
| Total (n=166) | Age (years) | |||
|---|---|---|---|---|
| <65 (n=63) | 65-74 (n=62) | ≥75 (n=41) | ||
| WBC (>10,000 /uL) | 114 (68.7%) | 45 (71.4%) | 42 (67.7%) | 27 (65.9%) |
| Hemoglobin (<12 g/dL) | 98 (59.0%) | 32 (50.8%) | 37 (59.7%) | 29 (70.7%) |
| Platelet (<130,000 /uL) | 43 (25.9%) | 9 (14.3%) | 25 (40.3%) | 9 (22.0%)∗ |
| PT (>15 sec) | 69 (41.6%) | 22 (34.9%) | 29 (46.8%) | 18 (43.9%) |
| AST (>36 IU/L) | 113 (68.1%) | 44 (69.8%) | 41 (66.1%) | 28 (68.3%) |
| ALT (>38 IU/L) | 97 (58.4%) | 41 (65.1%) | 35 (56.5%) | 21 (51.2%) |
| Bilirubin (>1.3 mg/dL) | 66 (39.8%) | 23 (36.5%) | 22 (35.5%) | 21 (51.2%) |
| Albumin (<3.0 g/dL) | 62 (37.3%) | 20 (31.7%) | 25 (40.3%) | 17 (41.5%) |
| BUN (>20 mg/dL) | 54 (32.5%) | 10 (15.9%) | 28 (45.2%) | 16 (39.0%)∗ |
| Creatinine (>1.2 mg/dL) | 32 (19.3%) | 6 (9.5%) | 17 (27.4%) | 9 (22.0%)∗ |
| ALP (>120 IU/L) | 115 (69.3%) | 47 (74.6%) | 41 (66.1%) | 27 (65.9%) |
| γ-GT (>40 IU/L) | 122 (73.5%) | 51 (81.0%) | 48 (77.4%) | 23 (56.1%)∗ |
Table 3.
Results of Blood and Abscess Culture in Pyogenic Liver Abscess
Table 4.
Comparison of Location, Size, and Number of Pyogenic Liver Abscess among Three Age Groups
| Total (n=166) | Age (years) | |||
|---|---|---|---|---|
| <65 (n=63) | 65-74 (n=62) | ≥75 (n=41) | ||
| Location | ||||
| Right | 113 (68.1%) | 53 (84.1%) | 39 (62.9%) | 21 (51.2%)∗ |
| Left | 45 (27.1%) | 7 (11.1%) | 21 (33.9%) | 17 (41.5%)∗ |
| Both | 8 (4.8%) | 3 (4.8%) | 2 (3.2%) | 3 (7.3%) |
| Size | ||||
| <5 cm | 59 (35.5%) | 24 (38.1%) | 22 (35.5%) | 13 (31.7%) |
| 5-10 cm | 97 (58.4%) | 35 (55.6%) | 36 (58.1%) | 26 (63.4%) |
| >10 cm | 10 (6.0%) | 4 (6.3%) | 4 (6.5%) | 2 (4.9%) |
| Number | ||||
| Solitary | 144 (86.7%) | 56 (88.9%) | 54 (87.1%) | 34 (82.9%) |
| Multiple | 22 (13.3%) | 7 (11.1%) | 8 (12.9%) | 7 (17.1%) |
Table 5.
Comparison of Treatment and Clinical Outcome among Three Age Groups
| Total (n=166) | Age (years) | |||
|---|---|---|---|---|
| <65 (n=63) | 65-74 (n=62) | ≥75 (n=41) | ||
| Length of stay (>3 wks) | 56 (33.7%) | 24 (38.1%) | 16 (25.8%) | 16 (39.0%) |
| PCD | 97 (58.4%) | 34 (54.0%) | 39 (62.9%) | 24 (58.5%) |
| PNA | 14 (8.4%) | 6 (9.5%) | 3 (4.8%) | 5 (12.2%) |
| Antibiotics only | 54 (32.5%) | 22 (34.9%) | 20 (32.3%) | 12 (29.3%) |
| Surgery | 1 (0.6%) | 1 (1.6%) | 0 | 0 |
| Improved | 150 (90.4%) | 58 (92.1%) | 60 (96.8%) | 32 (78.0%)∗ |
| Recurrence | 2 (1.2%) | 1 (1.6%) | 0 | 1 (2.4%) |
| Death | 8 (4.8%) | 2 (3.2%) | 2 (3.2%) | 4 (9.8%) |
Table 6.
Clinical Outcome by Treatment Methods
| Age (years) | ||||
|---|---|---|---|---|
| <65 (n=59) | 65-74 (n=62) | ≥75 (n=36) | ||
| Percutaneous drainage∗ | Improved | 37 (36.0%) | 40 (39.2%) | 24 (23.5%) |
| Death | 1 (20.0%) | 2 (40.0%) | 2 (40.0%) | |
| Antibiotics only | Improved | 20 (41.7%) | 20 (41.7%) | 8 (16.7%) |
| Death | 1 (33.3%) | 0 | 2 (66.7%) | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download