Abstract
The epithelial-mesenchymal transition (EMT) plays physiologic roles in the embryogenesis, wound healing, and tissue regeneration. In terms of pathological direction, it causes organ fibrosis, cancer development, progression, metastasis, and chemoresistance. Recently, the underlying mechanism of EMT and many kinds of EMT regulators have been identified. Pharmaceutical treatment strategies which target EMT pathway could be applied for the prevention of tissue fibrosis and cancer progression. In the field of gastroenterology, profuse evidences have been collected about the critical roles of EMT in cancers of the gastrointestinal tract, liver, and pancreas and hepatic fibrosis. However, EMT varies widely among cancer types, and much remains to be identified about the main regulators of EMT in a specific disease. In this review, we present recent research results regarding the roles of EMT in cancers and organic fibrosis, especially in the area of gastroenterology.
Go to : 
REFERENCES
1. Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009; 119:1429–1437.

2. Klymkowsky MW, Savagner P. Epithelial-mesenchymal transition: a cancer researcher's conceptual friend and foe. Am J Pathol. 2009; 174:1588–1593.
3. Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest. 2009; 119:1417–1419.

4. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009; 139:871–890.

5. Cordon-Cardo C, Prives C. At the crossroads of inflammation and tumorigenesis. J Exp Med. 1999; 190:1367–1370.

6. Ló pez-Novoa JM, Nieto MA. Inflammation and EMT: an alli-ance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009; 1:303–314.

7. Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009; 119:1438–1449.

9. Wells A, Yates C, Shepard CR. E-cadherin as an indicator of mesenchymal to epithelial reverting transitions during the metastatic seeding of disseminated carcinomas. Clin Exp Metastasis. 2008; 25:621–628.

10. Thompson EW, Williams ED. EMT and MET in carcino-S ma-clinical observations, regulatory pathways and new models. Clin Exp Metastasis. 2008; 25:591–592.
11. Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008; 133:704–715.

12. Kajiyama H, Shibata K, Terauchi M, et al. Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int J Oncol. 2007; 31:277–283.

13. Yang AD, Fan F, Camp ER, et al. Chronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell lines. Clin Cancer Res. 2006; 12:4147–4153.

14. Hiscox S, Jiang WG, Obermeier K, et al. Tamoxifen resistance in MCF7 cells promotes EMT-like behavior and in-volves modulation of beta-catenin phosphorylation. Int J Cancer. 2006; 118:290–301.
15. Hiscox S, Morgan L, Barrow D, Dutkowskil C, Wakeling A, Nicholson RI. Tamoxifen resistance in breast cancer cells is accompanied by an enhanced motile and invasive phenotype: inhibition by gefitinib (‘Iressa’, ZD1839). Clin Exp Metastasis. 2004; 21:201–212.

16. Fuchs BC, Fujii T, Dorfman JD, et al. Epithelial-to-mesenchymal transition and integrin-linked kinase mediate sensitivity to epidermal growth factor receptor inhibition in human hepatoma cells. Cancer Res. 2008; 68:2391–2399.

17. Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007; 39:305–318.

18. Iwatsuki M, Mimori K, Yokobori T, et al. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 2010; 101:293–299.

19. Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009; 19:156–172.
20. Bracken CP, Gregory PA, Khew-Goodall Y, Goodall GJ. The role of microRNAs in metastasis and epithelial-mesenchymal transition. Cell Mol Life Sci. 2009; 66:1682–1699.

21. Schneider M, Hansen JL, Sheikh SP. S100A4: a common me-diator of epithelial-mesenchymal transition, fibrosis and regeneration in diseases? J Mol Med. 2008; 86:507–522.

22. Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta. 2009; 1796:75–90.

23. Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 2007; 117:539–548.

24. Choi SS, Diehl AM. Epithelial-to-mesenchymal transitions in the liver. Hepatology. 2009; 50:2007–2013.

25. Aroeira LS, Aguilera A, Sá nchez-Tomero JA, et al. Epithelial to mesenchymal transition and peritoneal membrane failure in peritoneal dialysis patients: pathologic significance and potential therapeutic interventions. J Am Soc Nephrol. 2007; 18:2004–2013.

26. Natalwala A, Spychal R, Tselepis C. Epithelial-mesenchymal transition mediated tumourigenesis in the gastrointestinal tract. World J Gastroenterol. 2008; 14:3792–3797.

27. Uchikado Y, Natsugoe S, Okumura H, et al. Slug Expression in the E-cadherin preserved tumors is related to prognosis in patients with esophageal squamous cell carcinoma. Clin Cancer Res. 2005; 11:1174–1180.
28. Natsugoe S, Uchikado Y, Okumura H, et al. Snail plays a key role in E-cadherin preserved esophageal squamous cell carcinoma. Oncol Rep. 2007; 17:517–523.
29. Yuen HF, Chan YP, Wong ML, et al. Upregulation of Twist in esophageal squamous cell carcinoma is associated with neoplastic transformation and distant metastasis. J Clin Pathol. 2007; 60:510–514.
30. Jethwa P, Naqvi M, Hardy RG, et al. Overexpression of Slug is associated with malignant regression of esophageal adenocarcinoma. World J Gastroenterol. 2008; 14:1044–1052.
31. Rosivatz E, Becker KF, Kremmer E, et al. Expression and nuclear localization of Snail, an E-cadherin repressor, in adenocarcinomas of the upper gastrointestinal tract. Virchows Arch. 2006; 448:277–287.

32. Kim MA, Lee HS, Lee HE, Kim JH, Yang HK, Kim WH. Prognostic importance of epithelial-mesenchymal transition-related protein expression in gastric carcinoma. Histopathology. 2009; 54:442–451.

33. Rosivatz E, Becker I, Specht K, et al. Differential expression of the epithelial-mesenchymal transition regulators snail, SIP1, and twist in gastric cancer. Am J Pathol. 2002; 161:1881–1891.

34. Yang Z, Zhang X, Gang H, et al. Upregulation of gastric cancer cell invasion by Twist is accompanied by N-cadherin and fibronectin expression. Biochem Biophys Res Commun. 2007; 358:925–930.

35. Castro Alves C, Rosivatz E, Schott C, et al. Slug is overexpressed in gastric carcinomas and may act synergistically with SIP1 and Snail in the down-regulation of E-cadherin. J Pathol. 2007; 211:507–515.
36. Yan F, Cao H, Chaturvedi R, et al. Epidermal growth factor receptor activation protects gastric epithelial cells from Helicobacter pylori-induced apoptosis. Gastroenterology. 2009; 136:1297–1307.
37. Yin Y, Grabowska AM, Clarke PA, et al. Helicobacter pylori potentiates epithelial: mesenchymal transition in gastric cancer: links to soluble HB-EGF, gastrin and matrix metalloproteinase-7. Gut. 2010; 59:1037–1045.
38. Ohta H, Aoyagi K, Fukaya M, et al. Cross talk between hedgehog and epithelial-mesenchymal transition pathways in gastric pit cells and in diffuse-type gastric cancers. Br J Cancer. 2009; 100:389–398.

39. Wheeler JM, Kim HC, Efstathiou JA, Ilyas M, Mortensen NJ, Bodmer WF. Hypermethylation of the promoter region of the E-cadherin gene (CDH1) in sporadic and ulcerative colitis associated colorectal cancer. Gut. 2001; 48:367–371.

40. Roy HK, Smyrk TC, Koetsier J, Victor TA, Wali RK. The transcriptional repressor SNAIL is overexpressed in human colon cancer. Dig Dis Sci. 2005; 50:42–46.

41. Shioiri M, Shida T, Koda K, et al. Slug expression is an independent prognostic parameter for poor survival in colorectal carcinoma patients. Br J Cancer. 2006; 94:1816–1822.

42. Hong R, Choi DY, Lim SC, Suh CH, Kee KH, Lee MJ. The differential expressions of the epithelial-mesenchymal transition regulator, Slug and the cell adhesion molecule, E-cad-herin in colorectal adenocarcinoma. Korean J Pathol. 2008; 42:351–357.
43. Jang TJ, Jeon KH, Jung KH. Cyclooxygenase-2 expression is related to the epithelial-to-mesenchymal transition in human colon cancers. Yonsei Med J. 2009; 50:818–824.

44. Spaderna S, Schmalhofer O, Hlubek F, et al. A transient, EMT-linked loss of basement membranes indicates metastasis and poor survival in colorectal cancer. Gastroenterology. 2006; 131:830–840.

45. Yang MH, Chen CL, Chau GY, et al. Comprehensive analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. Hepatology. 2009; 50:1464–1474.

46. Jiao W, Miyazaki K, Kitajima Y. Inverse correlation between E-cadherin and Snail expression in hepatocellular carcinoma cell lines in vitro and in vivo. Br J Cancer. 2002; 86:98–101.

47. Sugimachi K, Tanaka S, Kameyama T, et al. Transcriptional repressor snail and progression of human hepatocellular carcinoma. Clin Cancer Res. 2003; 9:2657–2664.
48. Miyoshi A, Kitajima Y, Sumi K, et al. Snail and SIP1 in-crease cancer invasion by upregulating MMP family in hepatocellular carcinoma cells. Br J Cancer. 2004; 90:1265–1273.

49. Lee TK, Poon RT, Yuen AP, et al. Twist overexpression correlates with hepatocellular carcinoma metastasis through in-duction of epithelial-mesenchymal transition. Clin Cancer Res. 2006; 12:5369–5376.

50. Battaglia S, Benzoubir N, Nobilet S, et al. Liver cancer- derived hepatitis C virus core proteins shift TGF-beta responses from tumor suppression to epithelial-mesenchymal transition. PLoS ONE. 2009; 4:e4355.
51. Fransvea E, Angelotti U, Antonaci S, Giannelli G. Blocking transforming growth factor-beta upregulates E-cadherin and reduces migration and invasion of hepatocellular carcinoma cells. Hepatology. 2008; 47:1557–1566.

52. Mazzocca A, Fransvea E, Dituri F, Lupo L, Antonaci S, Giannelli G. Down-regulation of connective tissue growth factor by inhibition of transforming growth factor beta blocks the tumor-stroma cross-talk and tumor progression in hepatocellular carcinoma. Hepatology. 2010; 51:523–534.
53. Mazzocca A, Fransvea E, Lavezzari G, Antonaci S, Giannelli G. Inhibition of transforming growth factor beta receptor I kinase blocks hepatocellular carcinoma growth via neo-angio-genesis regulation. Hepatology. 2009; 50:1140–1151.
54. Fransvea E, Mazzocca A, Antonaci S, Giannelli G. Targeting transforming growth factor (TGF)-betaRI inhibits activation of beta1 integrin and blocks vascular invasion in hepatocellular carcinoma. Hepatology. 2009; 49:839–850.
55. Hotz B, Arndt M, Dullat S, Bhargava S, Buhr HJ, Hotz HG. Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer. Clin Cancer Res. 2007; 13:4769–4776.

56. Imamichi Y, Kö nig A, Gress T, Menke A. Collagen type I-induced Smad-interacting protein 1 expression downregulates E-cadherin in pancreatic cancer. Oncogene. 2007; 26:2381–2385.

57. Ohuchida K, Mizumoto K, Ohhashi S, et al. Twist, a novel oncogene, is upregulated in pancreatic cancer: clinical im-plication of Twist expression in pancreatic juice. Int J Cancer. 2007; 120:1634–1640.

58. Cates JM, Byrd RH, Fohn LE, Tatsas AD, Washington MK, Black CC. Epithelial-mesenchymal transition markers in pancreatic ductal adenocarcinoma. Pancreas. 2009; 38:e1–6.

59. Shah AN, Summy JM, Zhang J, Park SI, Parikh NU, Gallick GE. Development and characterization of gemcitabine-re-sistant pancreatic tumor cells. Ann Surg Oncol. 2007; 14:3629–3637.

60. Wang Z, Li Y, Kong D, et al. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009; 69:2400–2407.

61. Zeisberg M, Yang C, Martino M, et al. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007; 282:23337–23347.

62. Omenetti A, Porrello A, Jung Y, et al. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest. 2008; 118:3331–3342.

Go to : 
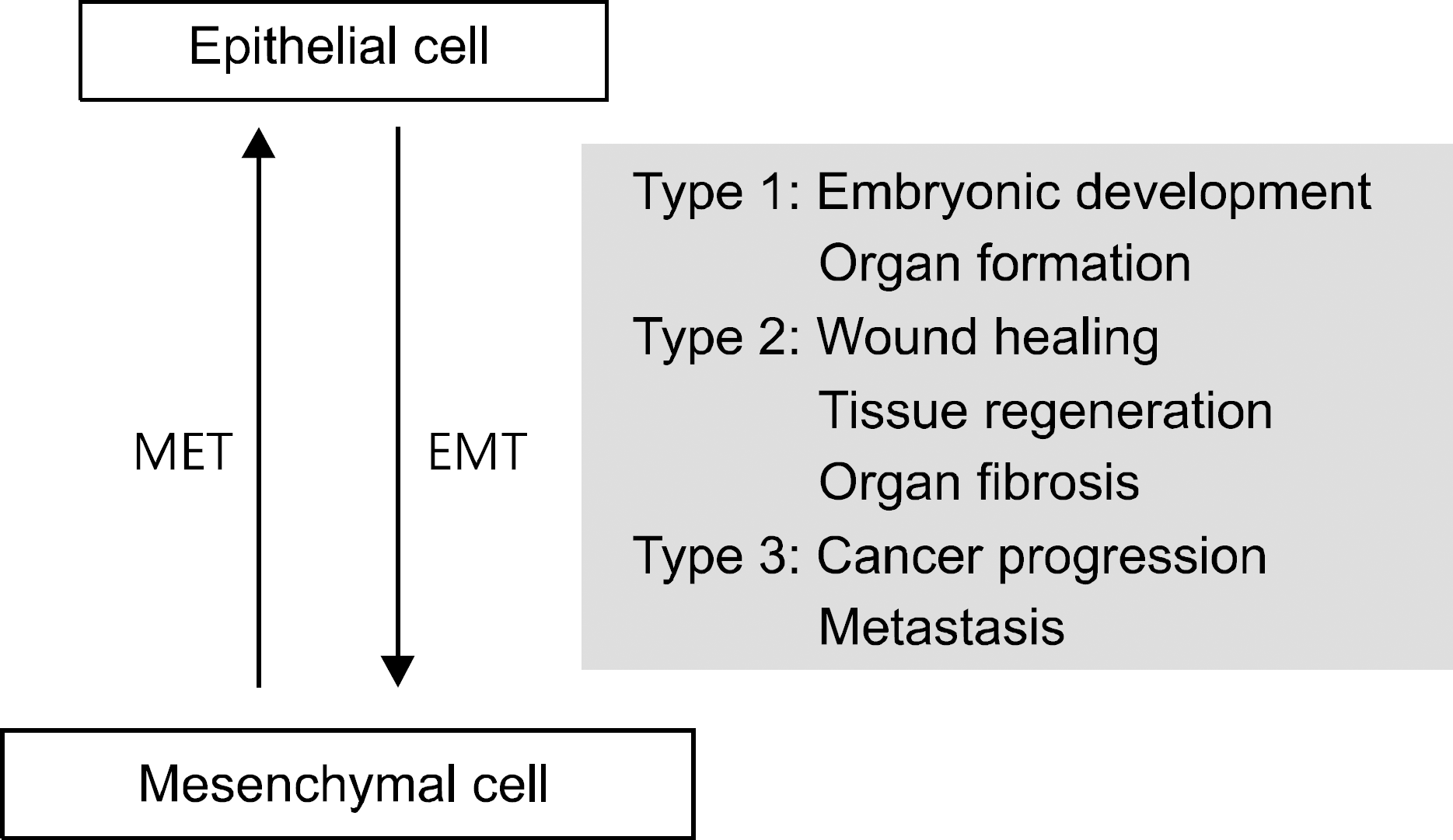 | Fig. 1.EMT and MET in normal and diseased condition. The EMT and MET is involved in embryonic development, organ formation, wound healing, tissue regeneration, organ fibrosis, cancer progression, and metastasis. EMT, epithelial-mesenchymal transition; MET, mesenchymal-epithelial transition. |
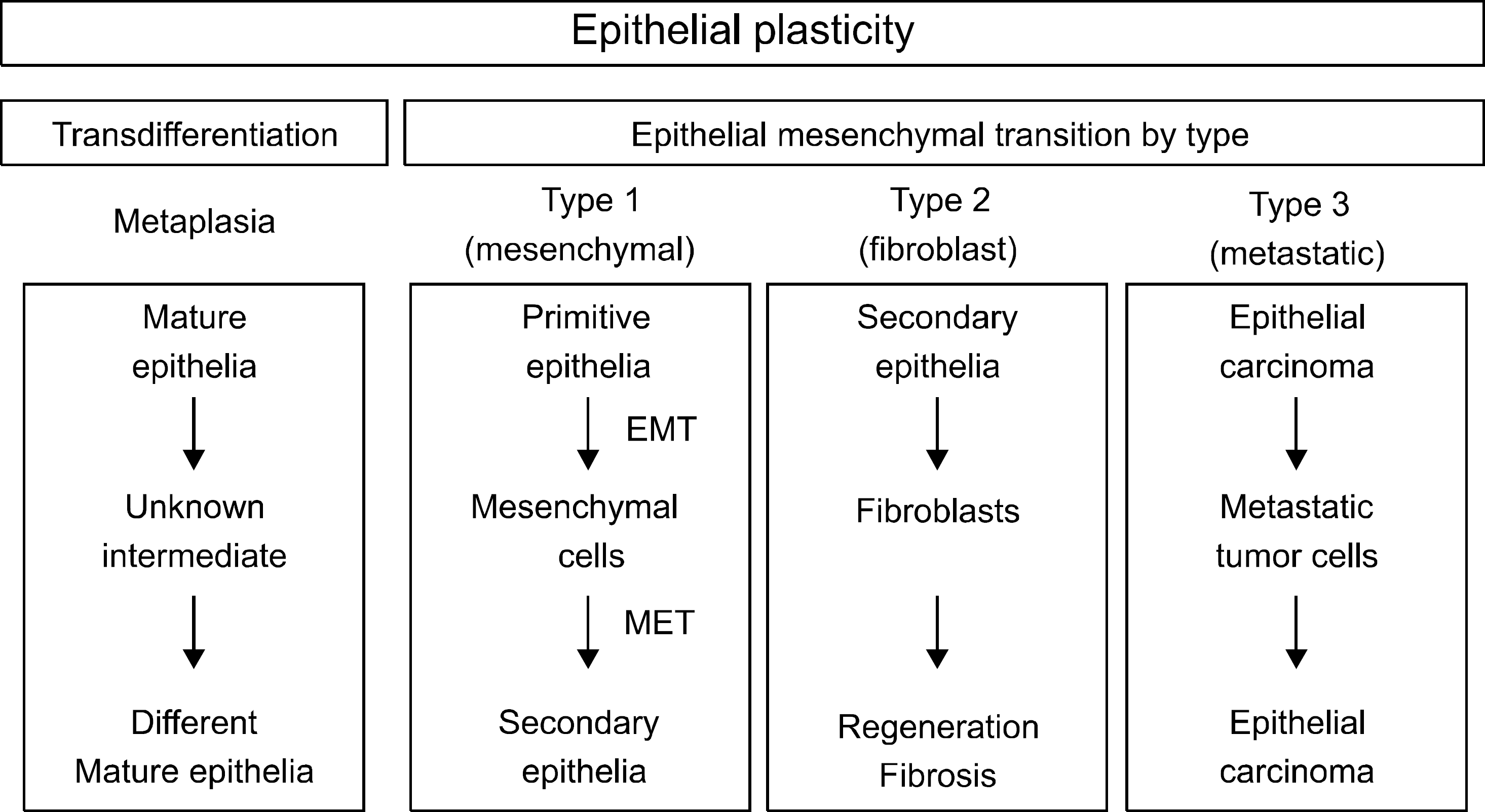 | Fig. 2.Epithelial cell plasticity can be viewed as a form of ei-ther transdifferentiation or EMT. Type 1 EMT is seen during development or organ formations. Type 2 EMT is seen when epithelial cells populate interstitial spaces with resident or inflam-mation-induced fibroblasts. Type 3 EMT is part of the metastatic process, whereby epithelial tumor cells detach a primary tumor nodule, migrate to a new tissue site, and reform as a secondary tumor nodule. (Adapted from Zeisberg and Neilson.1). |
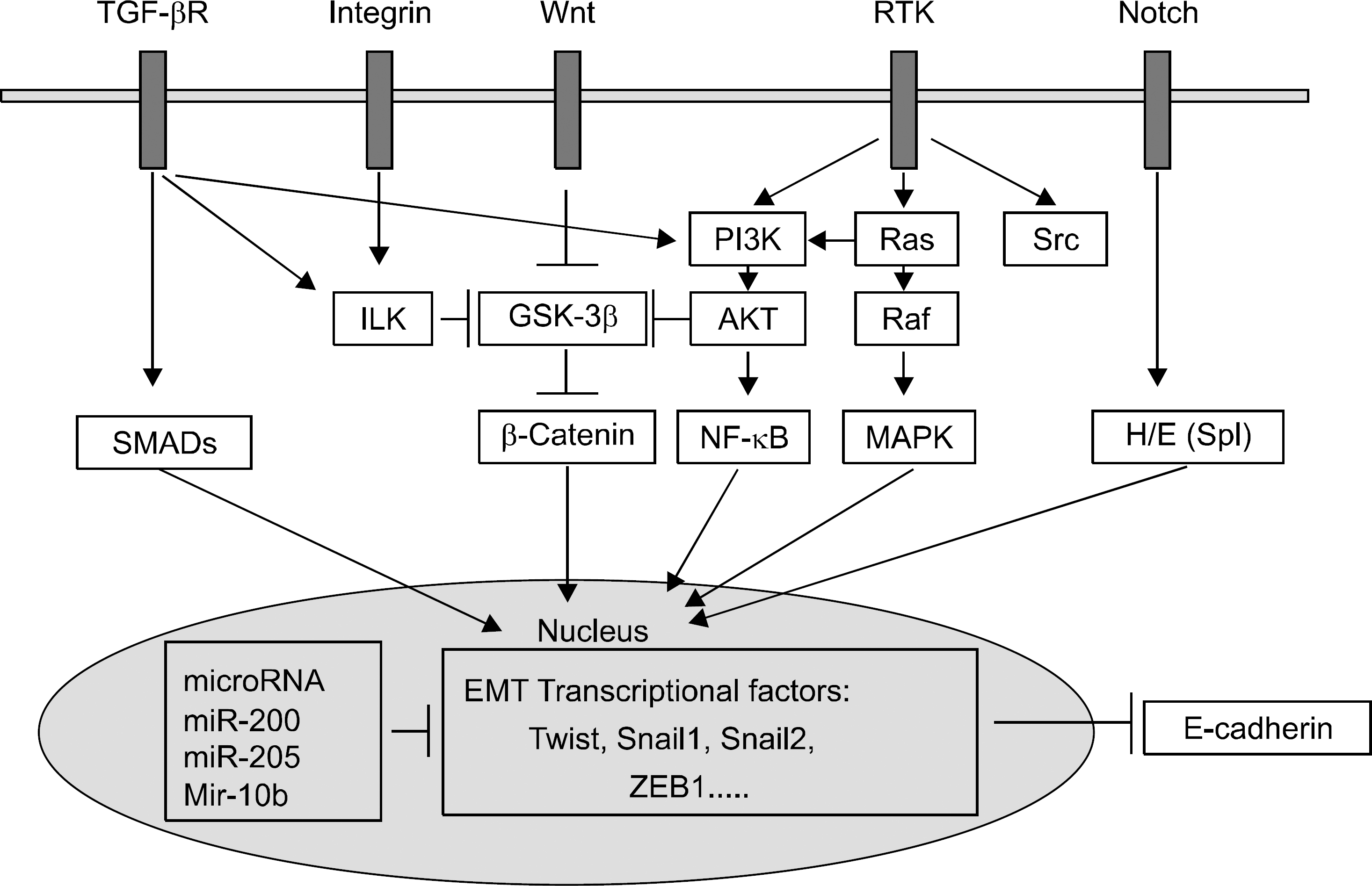 | Fig. 3.Signal pathways regulating the epithelial-mesenchymal transition (EMT). TGF-β signals toward the SMAD pathway or the PI3K/AKT axis. Wnt ligands block β-catenin degradation. Excess β-catenin enters the nucleus and upregulates slug and snail transcription. In integrin signaling, overexpression of ILK leads to nuclear translocation of β-catenin. Signals via RTK lead to EMT through the Ras-Raf-MAPK pathway or the PI3K/AKT pathway. AKT, serine/threonine kinase; GSK-3β, glycogen synthase kinase-3β; H/E (Spl), Hairy and enhancer of split; ILK, integrin-linked kinase; MAPK, mitogen-activated protein kinase; NF-κ B, nuclear factor-κ B; PI3K, phosphatidylinositol 3’ kinase; RTK, receptor tyrosine kinase; TGF-β R, transforming growth factor-β receptor; ZEB1, zinc finger E-box binding homeobox 1 (Adapted from Iwatsuki et al.18). |
Table 1.
Markers Regulating EMT Pathways
Table 2.
Studies about the EMT Regulators in Gastrointestinal Cancers




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download