Abstract
Mesothelioma is a rare aggressive tumor arising from the mesothelial cell and regarded as universally fatal disease with average survival around 1 year. The incidence rate is varied from one to forty per million in different countries and increasing by the year. The most common site of tumor origin is the pleura and only 20% to 33% of mesothelioma arise from the peritoneum. There are increasing reports of malignant mesothelioma with forty to fifty fatal cases per year in Korea. Histological studies with immunohistochemical stain is helpful for the diagnosis of peritoneal mesothelioma and imaging modality alone is not sufficient for diagnosis, so it is difficult to confirm diagnosis. A 64-year-old male patient was admitted to the hospital with a palpable mass on abdomen. The 6×6 cm sized huge mass was seen on the body of stomach adjacent to the peritoneum. We report a case of malignant peritoneal mesothelioma without evident exposure to asbestos, of which direct invasion to the gastric mucosa was confirmed by endoscopic biopsy and immunohistochemical stain.
Go to : 
REFERENCES
1. Hillerdal G. Malignant mesothelioma 1982: review of 4710 published cases. Br J Dis Chest. 1983; 77:321–343.

3. Sugarbaker PH, Acherman YI, Gonzalez-Moreno S, et al. Diagnosis and treatment of peritoneal mesothelioma: The Washington Cancer Institute experience. Semin Oncol. 2002; 29:51–61.

4. Deraco M, Bartlett D, Kusamura S, Baratti D. Consensus statement on peritoneal mesothelioma. J Surg Oncol. 2008; 98:268–272.

5. Kim HR, Ahn YS, Jung SH. Epidemiologic characteristics of malignant mesothelioma in Korea. J Korean Med Assoc. 2009; 52:449–455.

6. Marchevsky AM, Wick MR. Current controversies regarding the role of asbestos exposure in the causation of malignant mesothelioma: the need for an evidence-based approach to develop medicolegal guidelines. Ann Diagn Pathol. 2003; 7:321–332.

7. Husain AN, Colby TV, Ordóñ ez NG, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: a consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2009; 133:1317–1331.

9. Reuter K, Raptopoulos V, Reale F, et al. Diagnosis of peritoneal mesothelioma: computed tomography, sonography, and fine-needle aspiration biopsy. AJR Am J Roentgenol. 1983; 140:1189–1194.

11. Rake C, Gilham C, Hatch J, Darnton A, Hodgson J, Peto J. Occupational, domestic and environmental mesothelioma risks in the British population: a case-control study. Br J Cancer. 2009; 100:1175–1183.

13. Whitley NO, Brenner DE, Antman KH, Grant D, Aisner J. CT of peritoneal mesothelioma: analysis of eight cases. AJR Am J Roentgenol. 1982; 138:531–535.

14. Guest PJ, Reznek RH, Selleslag D, Geraghty R, Selvin M. Peritoneal mesothelioma: the role of computed tomography in diagnosis and follow up. Clin Radiol. 1992; 45:79–84.

15. Yan TD, Haveric N, Carmignani CP, Chang D, Sugarbaker PH. Abdominal computed tomography scans in the selection of patients with malignant peritoneal mesothelioma for com-prehensive treatment with cytoreductive surgery and perioperative intraperitoneal chemotherapy. Cancer. 2005; 103:839–849.

16. Attanoos RL, Webb R, Dojcinov SD, Gibbs AR. Value of mesothelial and epithelial antibodies in distinguishing diffuse peritoneal mesothelioma in females from serous papillary carcinoma of the ovary and peritoneum. Histopathology. 2002; 40:237–244.

17. Feldman AL, Libutti SK, Pingpank JF, et al. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol. 2003; 21:4560–4567.

18. Nonaka D, Kusamura S, Baratti D, et al. Diffuse malignant mesothelioma of the peritoneum: a clinicopathological study of 35 patients treated locoregionally at a single institution. Cancer. 2005; 104:2181–2188.
Go to : 
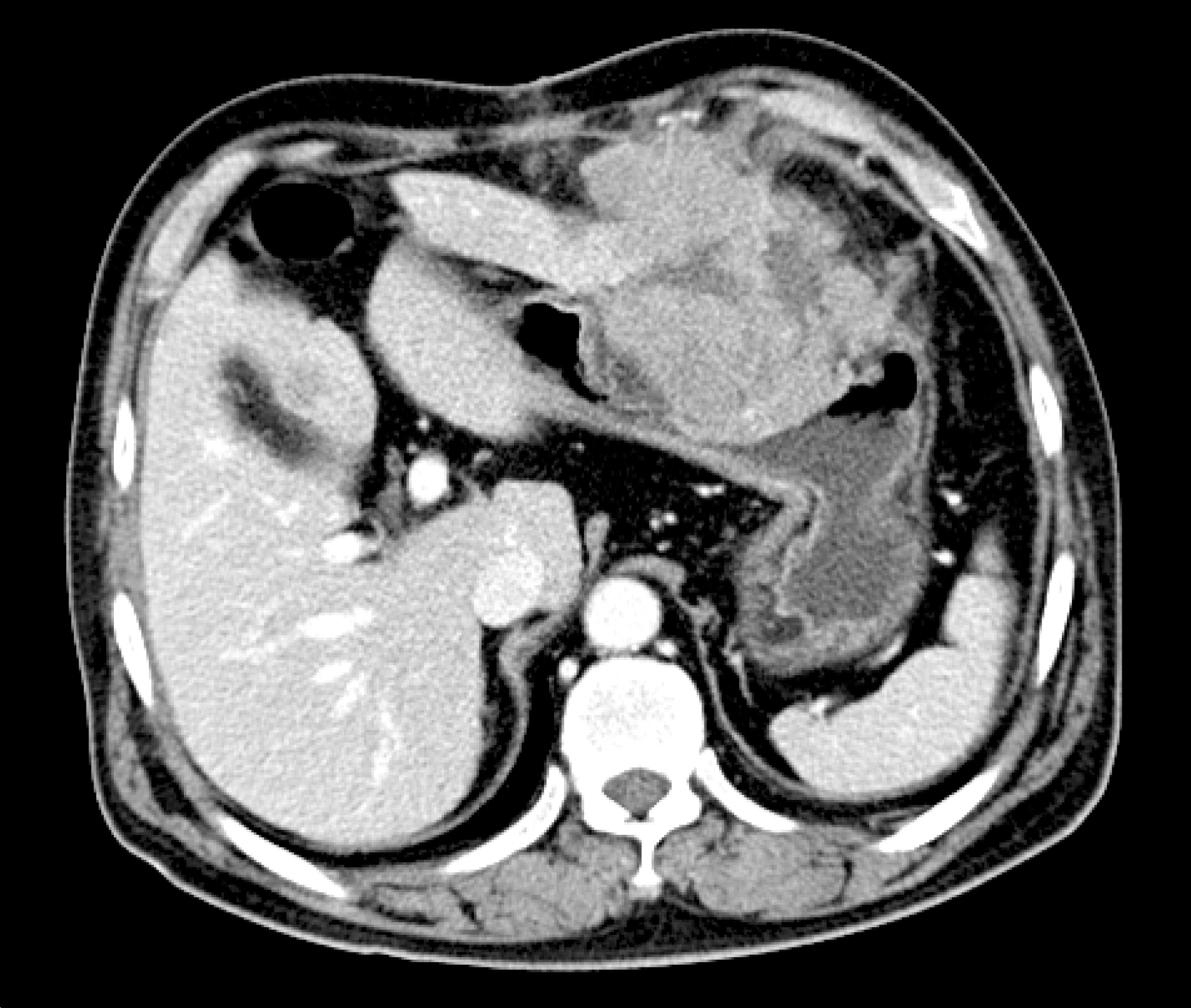
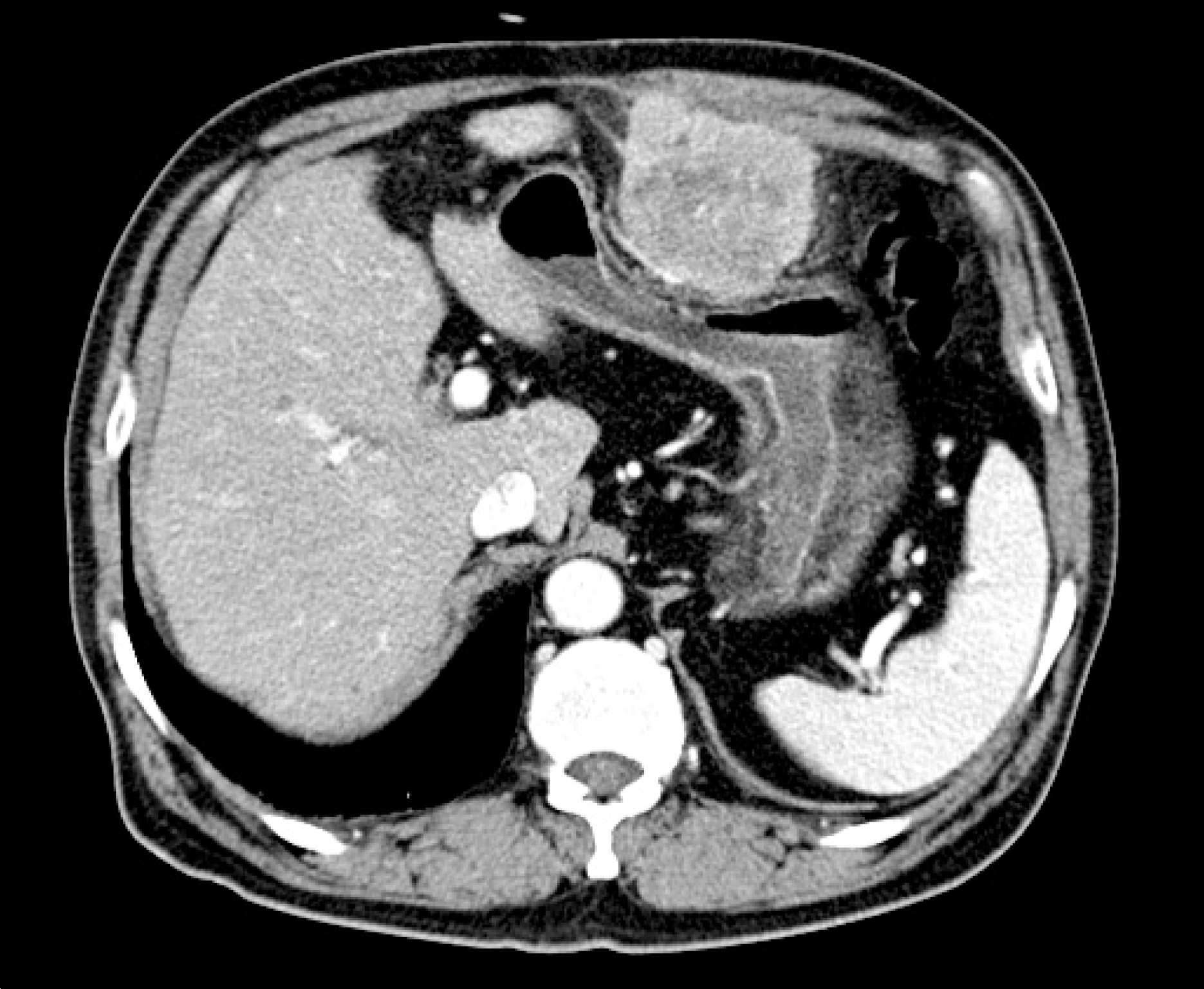 | Fig. 1.Computed tomography showed a 6×6 cm sized mass arising from the peritoneum and externally compressing the body of the stomach. |
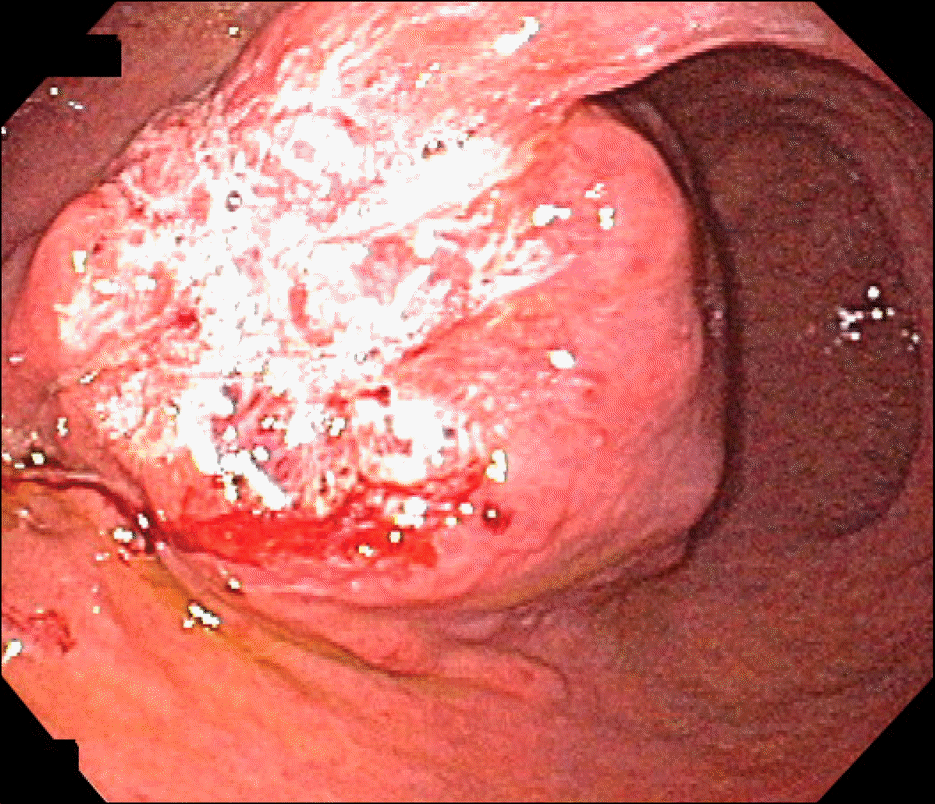 | Fig. 2.Endoscopic finding showed a firm subepithelial mass with irregular surface ulceration in anterior wall of the angle and antrum. |
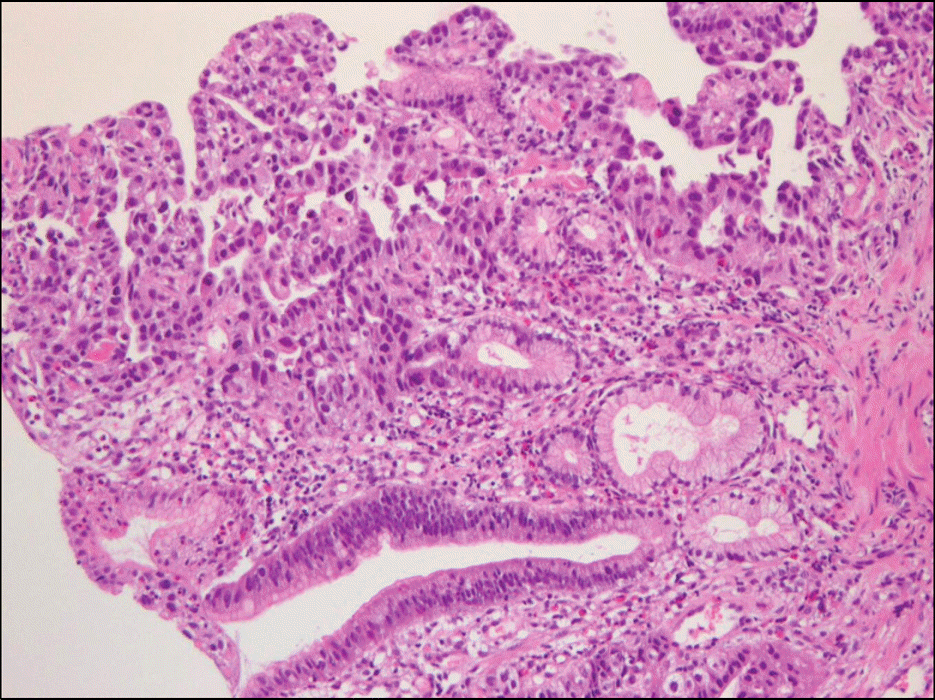 | Fig. 3.Histologic finding of endoscopic biopsy. The tumor cells were arranged in acinar and tubulopapillary pattern lined by pol-ygonal or cuboidal cells with fibrovascular cores and glandlike tubules (H&E stain, ×200). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download