Abstract
Background/Aims
Helicobacter pylori (H. pylori) transmission route is not yet clearly understood. Isolating H. pylori from stool, saliva, and vomitus is very difficult. However, H. pylori could be cultured from feces in the setting of rapid gastrointestinal tract transit. The aim of this study was to isolate H. pylori by culture and PCR in the rectum and terminal ileum during colonoscopy.
Methods
Twenty subjects with positive UBT (urea breath test) were included. We performed polymerase chain reaction (PCR) test and culture of H. pylori with the rectal fluid and terminal ileal fluid during colonoscopy.
Results
H. pylori was cultured with rectal fluid from 9 (45.0%) of 20 subjects and with ileal fluid from 11 (55.0%) of 20 subjects. H. pylori was a little more frequently cultured from the terminal ileal fluid than the rectal fluid without statistical significance (p>0.05). PCR test detected fla A (16/20, 80.0% and 17/20, 85.0%), 16S rRNA gene (16/20, 80.0% and 17/20, 85.0%), cag A (10/20, 50.0% and 12/20, 60.0%), and ure C (9/20, 45% and 11/20, 54.5%) from the rectal fluid and the terminal ileal fluid, respectively. The specificity and sensitivity of ure C were 100%.
Go to : 
REFERENCES
1. Li C, Ha T, Ferguson DA Jr, et al. A newly developed PCR assay of H. pylori in gastric biopsy, saliva, and feces. Evidence of high prevalenceof H. pylori in saliva supports oral transmission. Dig Dis Sci. 1996; 41:2142–2149.
2. Pytko‐ Polonczyk J, Konturek SJ, Karczewska E, Bielański W, Kaczmarczyk-Stachowska A. Oral cavity as permanent reservoir of Helicobacter pylori and potential source of reinfection. J Physiol Pharmacol. 1996; 47:121–129.
3. Gramley WA, Asghar A, Frierson HF Jr, Powell SM. Detection of Helicobacter pylori DNA in fecal samples from infected individuals. J Clin Microbiol. 1999; 37:2236–2240.
4. Thomas JE, Gibson GR, Darboe MK, Dale A, Weaver LT. Isolation of Helicobacter pylori from human faeces. Lancet. 1992; 340:1194–1195.
5. Kelly SM, Pitcher MC, Farmery SM, Gibson GR. Isolation of Helicobacter pylori from feces of patients with dyspepsia in the United Kingdom. Gastroenterology. 1994; 107:1671–1674.
6. Madinier IM, Fosse TM, Monteil RA. Oral carriage of Helicobacter pylori. a review. J Periodontol. 1997; 68:2–6.
7. Luman W, Alkout AM, Blackwell CC, Weir DM, Palmer KR. Helicobacter pylori in the mouth– negative isolation from dental plaque and saliva. Eur J Gastroenterol Hepatol. 1996; 8:11–14.
8. Lin SY, Jeng YS, Wang CK, et al. Polymerase chain reaction diagnosis of Helicobacter pylori in gastroduodenal diseases: comparison with culture and histopathological examinations. J Gastroenterol Hepatol. 1996; 11:286–289.
9. Falsafi T, Favaedi R, Mahjoub F, Najafi M. Application of stool-PCR test for diagnosis of Helicobacter pylori infection in children. World J Gastroenterol. 2009; 15:484–488.
10. Vaira D, Gatta L, Ricci C, Miglioli M. Review article: diagnosis of Helicobacter pylori infection. Aliment Pharmacol Ther. 2002; 16(suppl 1):S16–S23.
11. Mullis KB, Faloona FA. Specific synthesis of DNA in vitro via a polymerase‐ catalyzed chain reaction. Methods Enzymol. 1987; 155:335–350.
12. Tomb JF, White O, Kerlavage AR, et al. The complete ge-nome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997; 388:539–547.
13. Engstrand L, Nguyen AM, Graham DY, el-Zaatari FA. Reverse transcription and polymerase chain reaction amplifi-cation of rRNA for detection of helicobacter species. J Clin Microbiol. 1992; 30:2295–2301.
14. Peek RM Jr, Miller GG, Tham KT, et al. Detection of Helicobacter pylori gene expression in human gastric mucosa. J Clin Microbiol. 1995; 33:28–32.
15. Kabir S. Detection of Helicobacter pylori in faeces by culture, PCR and enzyme immunoassay. J Med Microbiol. 2001; 50:1021–1029.
16. Sicinschi LA, Correa P, Bravob LE, Schneidera BG. Detection and typing of Helicobacter pylori cagA/vacA genes by radioactive, one-step polymerase chain reaction in stool samples from children. J Microbiol Methods. 2003; 52:197–207.
17. Kim GH, Ok CM, Yu YI, et al. Detection of Helicobacter pylori in gastric biopsy specimen by polymerase chain reaction. Korean J Med. 1997; 52:584–592.
18. Namavar F, Roosendaal R, Kuipers EJ, et al. Presence of Helicobacter pylori in the oral cavity, oesophagus, stomach and faeces of patients with gastritis. Eur J Clin Microbiol Infect Dis. 1995; 14:234–237.
Go to : 
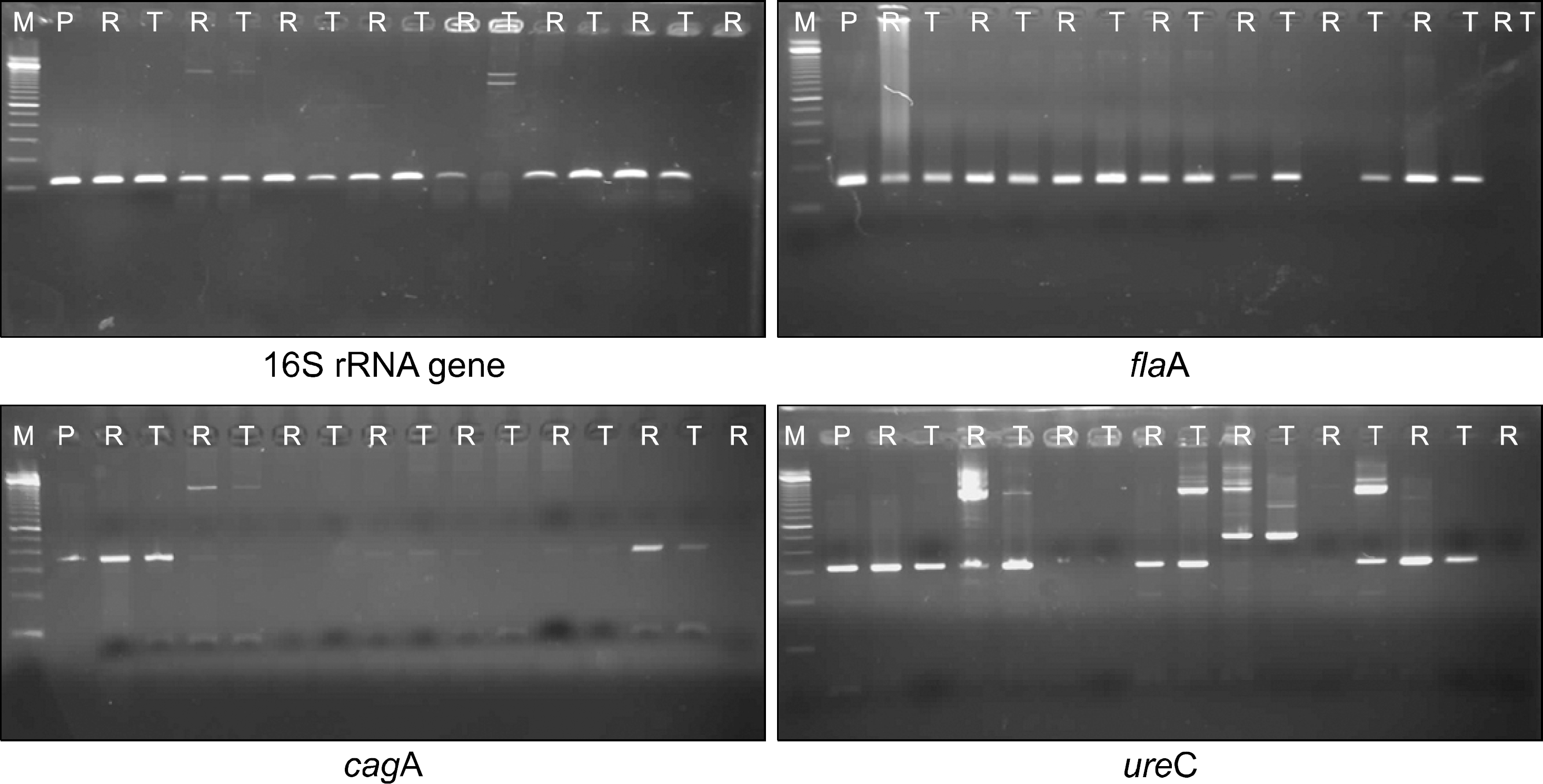 | Fig. 1.Detection of H. pylori DNA in the rectal fluid and terminal ileal fluid by PCR. M, marker; P, positive control; R, rectal fluid; T, terminal ileal fluid. |
Table 1.
Summary of PCR and Culture Results from Rectal Fluid and Terminal Ileal Fluid of 20 Subjects Infected with Helicobacter pylori
Table 2.
Comparison of Different H. pylori DNA in Culture Positive Specimens from the Rectal Fluid
| cag A | fla A | 16s rRNA | ure C | |
|---|---|---|---|---|
| Sensitivity | 88.9 | 100 | 100 | 100 |
| Specificity | 81.8 | 36.4 | 36.4 | 100 |
| PPV | 80 | 56.3 | 56.3 | 100 |
| NPV | 90 | 100 | 100 | 100 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download