Abstract
Current understanding of the pathophysiology of portal hypertension has resulted in therapeutic approaches aimed at correcting the increased splanchnic blood flow and some of which have been already used in clinical practice. Recently new perspectives opened and erstwhile paradigm has been changed to focus on increased resistance to portal blood flow and the formation of portosystemic collateralization. Several studies revealed the clear-cut mechanisms of hepatic endothelial dysfunction and abnormal angiogenesis contributing to the development of portal hypertension. Thus the modulations of hyperdynamic circulation or angiogenesis seem to be valuable therapeutic targets. In the current review update, we discuss the multidisciplinary management of modulating hepatic vascular resistance and abnormal angiogenesis associated with portal hypertension. However, these new pharmacological approaches are still under investigation and widescale clinical application are needed to develop effective strategies.
REFERENCES
1. D'Amico G, Garcia-Pagan JC, Luca A, Bosch J. Hepatic Vein Pressure Gradient Reduction and Prevention of Variceal Bleeding in Cirrhosis: A Systematic Review. Gastroenterology. 2006; 131:1611–1624.
2. Bosch J, Berzigotti A, Garcia-Pagan JC, Abraldes JG. The management of portal hypertension: Rational basis, available treatments and future options. J Hepatol. 2008; 48(suppl 1):S68–S92.

3. Rockey DC, Weisiger RA. Endothelin induced contractility of stellate cells from normal and cirrhotic rat liver: Implications for regulation of portal pressure and resistance. Hepatology. 1996; 24:233–240.

4. Pinzani M, Gentilini P. Biology of hepatic stellate cells and their possible relevance in the pathogenesis of portal hypertension in cirrhosis. Semin Liver Dis. 1999; 19:397–410.

5. Wiest R, Groszmann RJ. The paradox of nitric oxide in cirrhosis and portal hypertension: too much, not enough. Hepatology. 2002; 35:478–491.

6. Herná ndez-Guerra M, Garcia-Pagá n JC, Bosch J. Increased hepatic resistance: a new target in the pharmacologic therapy of portal hypertension. J Clin Gastroenterol. 2005; 39(4 suppl 2):S131–S137.
7. Vorobioff J, Bredfeldt JE, Groszmann RJ. Hyperdynamic circulation in portal-hypertensive rat model: a primary factor for maintenance of chronic portal hypertension. Am J Physiol. 1983; 244:G52–G57.

8. Benoit JN, Barrowman JA, Harper SL, Kvietys PR, Granger DN. Role of humoral factors in the intestinal hyperemia associated with chronic portal hypertension. Am J Physiol. 1984; 247:G486–G493.

9. Pizcueta P, Piqué JM, Ferná ndez M, et al. Modulation of the hyperdynamic circulation of cirrhotic rats by nitric oxide inhibition. Gastroenterology. 1992; 103:1909–1915.

10. Wiest R, Groszmann RJ. Nitric oxide and portal hypertension: its role in the regulation of intrahepatic and splanchnic vascular resistance. Semin Liver Dis. 1999; 19:411–426.

11. Garcia-Pagan JC, De Gottardi A, Bosch J. Review article: the modern management of portal hypertension–primary and secondary prophylaxis of variceal bleeding in cirrhotic patients. Aliment Pharmacol Ther. 2008; 28:178–186.
12. Ferná ndez M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J. Angiogenesis in liver disease. J Hepatol. 2009; 50:604–620.
13. Zhang JX, Pegoli W Jr, Clemens MG. Endothelin-1 induces direct constriction of hepatic sinusoids. Am J Physiol. 1994; 266:G624–G632.

14. Kawada N, Tran-Thi TA, Klein H, Decker K. The contraction of hepatic stellate (Ito) cells stimulated with vasoactive substances. Possible involvement of endothelin 1 and nitric oxide in the regulation of the sinusoidal tonus. Eur J Biochem. 1993; 213:815–823.

15. Povero D, Busletta C, Novo E, et al. Liver fibrosis: a dynamic and potentially reversible process. Histol Histopathol. 2010; 25:1075–1091.
16. Fallowfield JA, Kendall TJ, Iredale JP. Reversal of fibrosis: no longer a pipe dream? Clin Liver Dis. 2006; 10:481–497.

17. Shiratori Y, Imazeki F, Moriyama M, et al. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000; 132:517–524.

18. Rincon D, Ripoll C, Lo lacono O, et al. Antiviral therapy decreases hepatic venous pressure gradient in patients with chronic hepatitis C and advanced fibrosis. Am J Gastroenterol. 2006; 101:2269–2274.

19. Paik YH, Kim JK, Lee JI, et al. Celecoxib induces hepatic stellate cell apoptosis through inhibition of Akt activation and suppresses hepatic fibrosis in rats. Gut. 2009; 58:1517–1527.

20. Terai S, Yamamoto N, Omori K, Sakaida I, Okita K. A new cell therapy using bone marrow cells to repair damaged liver. J Gastroenterol. 2002; 37:162–163.

21. Sakaida I, Terai S, Yamamoto N, et al. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004; 40:1304–1311.

22. Segawa M, Sakaida I. Diagnosis and treatment of portal hypertension. Hepatol Res. 2009; 39:1039–1043.

23. Lee JS, Kim JH. The role of activated hepatic stellate cells in liver fibrosis, portal hypertension and cancer angiogenesis. Korean J Hepatol. 2007; 13:309–319.

24. Thimgan MS, Yee HF Jr. Quantitation of rat hepatic stellate cell contraction: stellate cells' contribution to sinusoidal resistance. Am J Physiol. 1999; 277:G137–G143.
25. Harrison DG. Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest. 1997; 100:2153–2157.

26. Harrison DG. Endothelial function and oxidant stress. Clin Cardiol. 1997; 20(suppl II):II–11. -II-17.

27. Gupta TK, Toruner M, Chung MK, Groszmann RJ. Endothelial dysfunction and decreased production of nitric oxide in the intrahepatic microcirculation of cirrhotic rats. Hepatology. 1998; 28:926–931.

28. Graupera M, Garcia-Pagan JC, Pares M, et al. Cyclooxygen-ase-1 inhibition corrects endothelial dysfunction in cirrhotic rat livers. J Hepatol. 2003; 39:515–521.

29. Gracia-Sancho J, Laviñ a B, Rodrí guez-Vilarrupla A, Garcí a- Calderó H, Bosch J, Garcí a-Pagá n JC. Enhanced vasocon-strictor prostanoid production by sinusoidal endothelial cells increases portal perfusion pressure in cirrhotic rat livers. J Hepatol. 2007; 47:220–227.

30. Steib CJ, Gerbes AL, Bystron M, et al. Kupffer cell activation in normal and fibrotic livers increases portal pressure via thromboxane A(2). J Hepatol. 2007; 47:228–238.

31. Mittal MK, Gupta TK, Lee FY, Sieber CC, Groszmann RJ. Nitric oxide modulates hepatic vascular tone in normal rat liver. Am J Physiol. 1994; 267:G416–G422.

32. Miller DR, Collier JM, Billings RE. Protein tyrosine kinase activity regulates nitric oxide synthase induction in rat he-patocytes. Am J Physiol. 1997; 272:G207–G214.

33. Shah V, Toruner M, Haddad F, et al. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology. 1999; 117:1222–1228.

34. Lee JS, Semela D, Iredale J, Shah VH. Sinusoidal remodeling and angiogenesis: a new function for the liver-specific peri-cyte? Hepatology. 2007; 45:817–825.

35. Abraldes JG, Rodrií guez-Vilarrupla A, Graupera M, et al. Simvastatin treatment improves liver sinusoidal endothelial dysfunction in CCl4 cirrhotic rats. J Hepatol. 2007; 46:1040–1046.

36. Zafra C, Abraldes JG, Turnes J, et al. Simvastatin enhances hepatic nitric oxide production and decreases the hepatic vascular tone in patients with cirrhosis. Gastroenterology. 2004; 126:749–755.

37. Laviñ a B, Gracia-Sancho J, Rodrí guez-Vilarrupla A, et al. Superoxide dismutase gene transfer reduces portal pressure in CCl4 cirrhotic rats with portal hypertension. Gut. 2009; 58:118–125.

38. Herná ndez-Guerra M, Garcí a-Pagá n JC, Turnes J, et al. Ascorbic acid improves the intrahepatic endothelial dysfunction of patients with cirrhosis and portal hypertension. Hepatology. 2006; 43:485–491.

39. Matei V, Rodrí guez-Vilarrupla A, Deulofeu R, et al. The eNOS cofactor tetrahydrobiopterin improves endothelial dysfunction in livers of rats with CCl4 cirrhosis. Hepatology. 2006; 44:44–52.

40. Garcia-Pagan JC, Villanueva C, Vila MC, et al. Isosorbide mononitrate in the prevention of first variceal bleed in patients who cannot receive beta-blockers. Gastroenterology. 2001; 121:908–914.
41. Angelico M, Carli L, Piat C, Gentile S, Capocaccia L. Effects of isosorbide-5-mononitrate compared with propranolol on first bleeding and longterm survival in cirrhosis. Gastroenterology. 1997; 113:1632–1639.

42. Fiorucci S, Antonelli E, Morelli O, et al. NCX-1000, a NO-releasing derivative of ursodeoxycholic acid, selectively delivers NO to the liver and protects against development of portal hypertension. Proc Natl Acad Sci U S A. 2001; 98:8897–8902.

43. Fiorucci S, Antonelli E, Brancaleone V, et al. NCX-1000, a nitric oxide-releasing derivative of ursodeoxycholic acid, ameliorates portal hypertension and lowers norepinephrine-induced intrahepatic resistance in the isolated and perfused rat liver. J Hepatol. 2003; 39:932–939.

44. Berzigotti A, Bellot P, De Gottardi A, et al. NCX-1000, a nitric oxide-releasing derivative of UCDA, does not decrease portal pressure in patients with cirrhosis: Rresults of a randomized, double-blind, dose-escalating study. Am J Gastroenterol. 2010; 105:1094–1101.
45. Van De Casteele M, Omasta A, Janssens S, et al. In vivo gene transfer of endothelial nitric oxide synthase decreases portal pressure in anaesthetised carbon tetrachloride cirrhotic rats. Gut. 2002; 51:440–445.

46. Shah V, Chen AF, Cao S, et al. Gene transfer of recombinant endothelial nitric oxide synthase to liver in vivo and in vitro. Am J Physiol Gastrointest Liver Physiol. 2000; 279:G1023–G1030.

47. Yu Q, Shao R, Qian HS, George SE, Rockey DC. Gene transfer of the neuronal NO synthase isoform to cirrhotic rat liver ameliorates portal hypertension. J Clin Invest. 2000; 105:741–748.

48. Morales-Ruiz M, Cejudo-Martí n P, Fernaá ndez-Varo G, et al. Transduction of the liver with activated Akt normalizes portal pressure in cirrhotic rats. Gastroenterology. 2003; 125:522–531.

49. Dangas G, Smith DA, Unger AH, et al. Pravastatin: An an-tithrombotic effect independent of the cholesterol-lowering effect. Thromb Haemost. 2000; 83:688–692.

51. Kureishi Y, Luo Z, Shiojima I, et al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000; 6:1004–1010.

52. Bates K, Ruggeroli CE, Goldman S, Gaballa MA. Simvastatin restores endothelial NO-mediated vasorelaxation in large ar-teries after myocardial infarction. Am J Physiol Heart Circ Physiol. 2002; 283:H768–H775.
53. Laufs U, Gertz K, Dirnagl U, Bö hm M, Nickenig G, Endres M. Rosuvastatin, a new HMG-CoA reductase inhibitor, upre-gulates endothelial nitric oxide synthase and protects from ischemic stroke in mice. Brain Res. 2002; 942:23–30.

54. Kalinowski L, Dobrucki LW, Brovkovych V, Malinski T. Increased nitric oxide bioavailability in endothelial cells con-tributes to the pleiotropic effect of cerivastatin. Circulation. 2002; 105:933–938.

55. Fulton D, Gratton JP, McCabe TJ, et al. Regulation of endo-thelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999; 399:597–601.

56. Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998; 97:1129–1135.

57. Trebicka J, Hennenberg M, Laleman W, et al. Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/Rho-kinase and activation of endothelial nitric oxide synthase. Hepatology. 2007; 46:242–253.

58. Abraldes JG, Albillos A, Bañ ares R, et al. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology. 2009; 136:1651–1658.

59. Mastai R, Bosch J, Navasa M, et al. Effects of alpha-adrenergic stimulation and beta-adrenergic blockade on azygos blood flow and splanchnic haemodynamics in patients with cirrhosis. J Hepatol. 1987; 4:71–79.

60. Bosch J, Abraldes JG, Ferná ndez M, Garcí a-Pagá n JC. Hepatic endothelial dysfunction and abnormal angiogenesis: new targets in the treatment of portal hypertension. J Hepatol. 2010; 53:558–567.

61. Moreno M, Ramalho LN, Sancho-Bru P, et al. Atorvastatin attenuates angiotensin II-induced inflammatory actions in the liver. Am J Physiol Gastrointest Liver Physiol. 2009; 296:G147–G156.

62. Glynn RJ, Danielson E, Fonseca FA, et al. A randomized trial of rosuvastatin in the prevention of venous thromboem-bolism. N Engl J Med. 2009; 360:1851–1861.

63. Francoz C, Belghiti J, Vilgrain V, et al. Splanchnic vein thrombosis in candidates for liver transplantation: Usefulness of screening and anticoagulation. Gut. 2005; 54:691–697.

64. Zocco MA, Di Stasio E, De Cristofaro R, et al. Thrombotic risk factors in patients with liver cirrhosis: correlation with MELD scoring system and portal vein thrombosis development. J Hepatol. 2009; 51:682–689.

65. Miyamoto T, Ogino N, Yamamoto S, Hayaishi O. Purificat-ion of prostaglandin endoperoxide synthetase from bovine ve-sicular gland microsomes. J Biol Chem. 1976; 251:2629–2636.

67. Matei V, Rodrí guez-Vilarrupla A, Deulofeu R, et al. Three- day tetrahydrobiopterin therapy increases in vivo hepatic NOS activity and reduces portal pressure in CCl4 cirrhotic rats. J Hepatol. 2008; 49:192–197.
68. Gracia-Sancho J, Laviñ a B, Rodrí guez-Vilarrupla A, et al. Increased oxidative stress in cirrhotic rat livers: A potential mechanism contributing to reduced nitric oxide bioavailability. Hepatology. 2008; 47:1248–1256.

69. Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ Res. 2000; 87:840–844.

70. Mejias M, Garci-Pras E, Tiani C, Miquel R, Bosch J, Fernandez M. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology. 2009; 49:1245–1256.

71. Fernandez M, Mejias M, Garcia-Pras E, Mendez R, Garcia- Pagan JC, Bosch J. Reversal of portal hypertension and hyperdynamic splanchnic circulation by combined vascular endothelial growth factor and platelet-derived growth factor blockade in rats. Hepatology. 2007; 46:1208–1217.

72. Tugues S, Fernandez-Varo G, Muñ oz-Luque J, et al. Antiangiogenic treatment with sunitinib ameliorates inflammatory in-filtrate, fibrosis, and portal pressure in cirrhotic rats. Hepatology. 2007; 46:1919–1926.

73. Shah VH, Bruix J. Antiangiogenic therapy: Not just for cancer anymore? Hepatology. 2009; 49:1066–1068.

74. Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W, Practice Guidelines Committee of the American Association for the Study of Liver Diseases, Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007; 46:922–938.
75. Bosch J. Carvedilol for portal hypertension in patients with cirrhosis. Hepatology. 2010; 51:2214–2218.

76. Bosch J, Arroyo V, Betriu A, et al. Hepatic hemodynamics and the renin-angiotensin-aldosterone system in cirrhosis. Gastroenterology. 1980; 78:92–99.

77. Tandon P, Abraldes JG, Berzigotti A, Garcia-Pagan JC, Bosch J. Renin-angiotensin-aldosterone inhibitors in the reduction of portal pressure: a systematic review and meta analysis. J Hepatol. 2010; 53:273–282.
Fig. 1.
New paradigm for the treatment of portal hypertension. Current treatments are based on the use of drugs that decrease blood flow by attenuating the splanchnic vasodilatation (old paradigm), but new treatments aim at correcting the increased hepatic vascular resistance and the formation of portal-systemic collaterals. (modified from Bosch et al., J of Hepatology 2010).
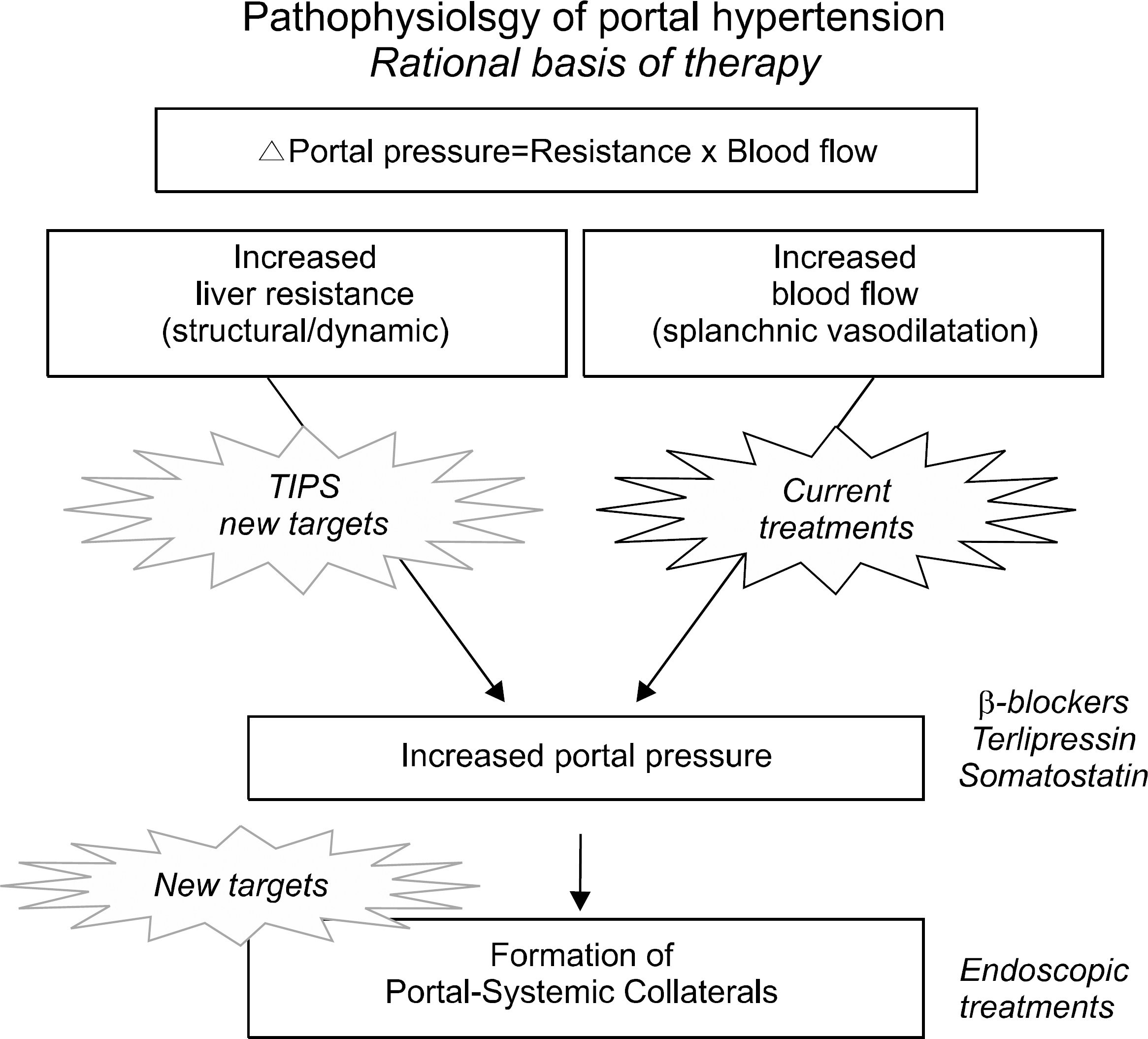
Fig. 2.
Mechanisms mediating the decrease in hepatic resistance by statins. Statins increase eNOS expression and activity. The most immediate effect of statins on endothelial NO production is an increase in eNOS phosphorylation, with subsequent increased activity. This is mediated by the activation of the phosphatidyl inositol 3-kinase (PI3K)/Akt pathway that leads to an increase in Akt phosphorylation with ensuing eNOS phosphorylation. Statins reduce the expression of the eNOS inhibitory protein caveolin-1 and increase the interaction of eNOS with its stimulatory protein Hsp90. These effects occur slower than the PI3K/Akt pathway activation. Statins also increase the expression of GTP cyclohydrolase I (GTPCH), the rate-limiting enzyme for de novo synthesis of tetrahydrobiopterin (BH4), a cofactor that increases eNOS activity by preventing eNOS uncoupling and, thus, superoxide generation. Statins also upregulate eNOS expression by increasing eNOS mRNA stability. Lastly, statins inhibit hepatic RhoA/Rho kinase signalling, which increases eNOS expression and decreases hepatic stellate cell (HSC) contractility (modified from Bosch et al., J of Hepatology 2010).
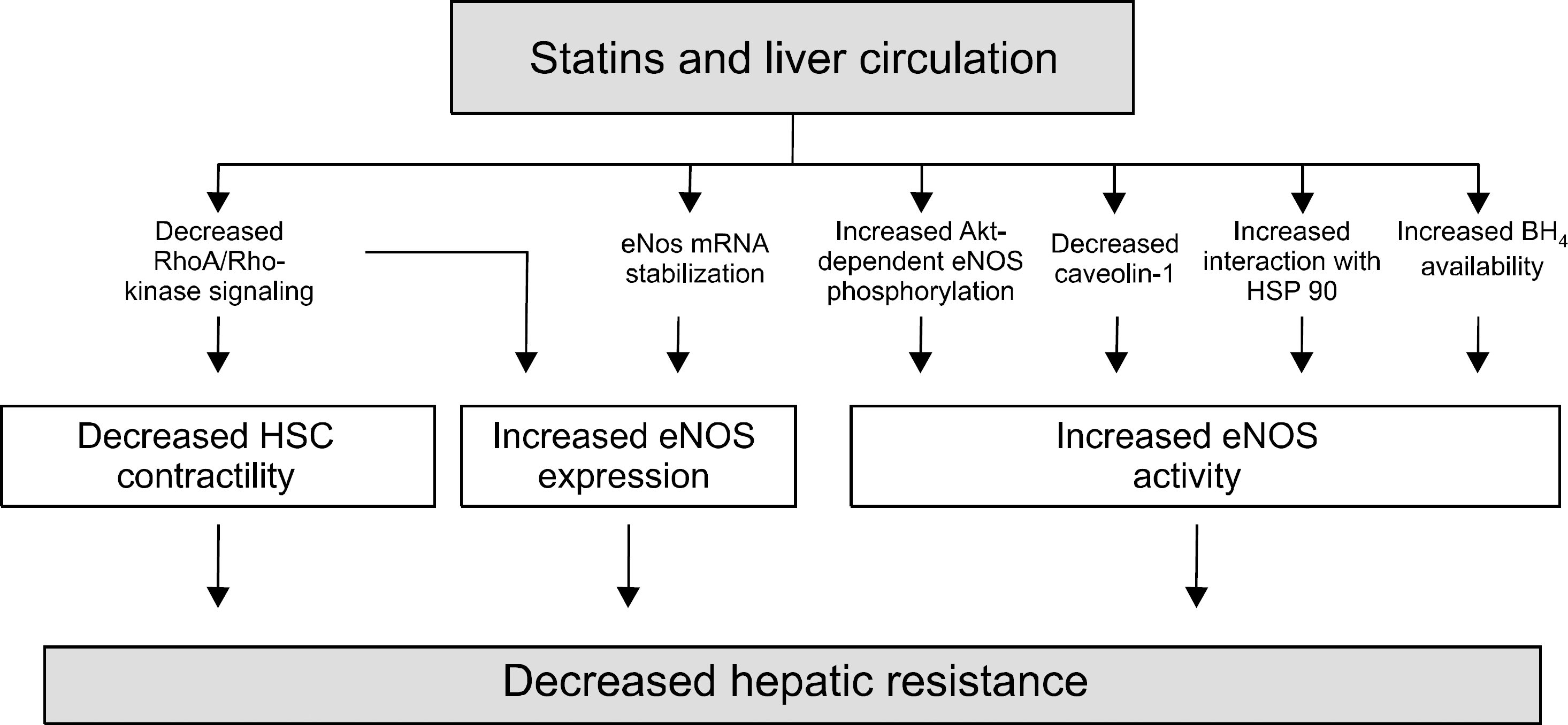
Fig. 3.
Double-blind randomized controlled trial of simvastatin vs. placebo for portal hypertension in patients with cirrhosis. (A) HVPG decreased significantly in patients receiving simvastatin, but not in those receiving placebo. (B) In the group of patients treated with simvastatin, the decrease in portal pressure was observed both in patients under no treatment and in patients under continuous propranolol administration, suggesting an additive effect with non-selective beta-blockers (modified from Abraldes et al., Gastroenterology 2009). HVPG, hepatic venous pressure gradient.
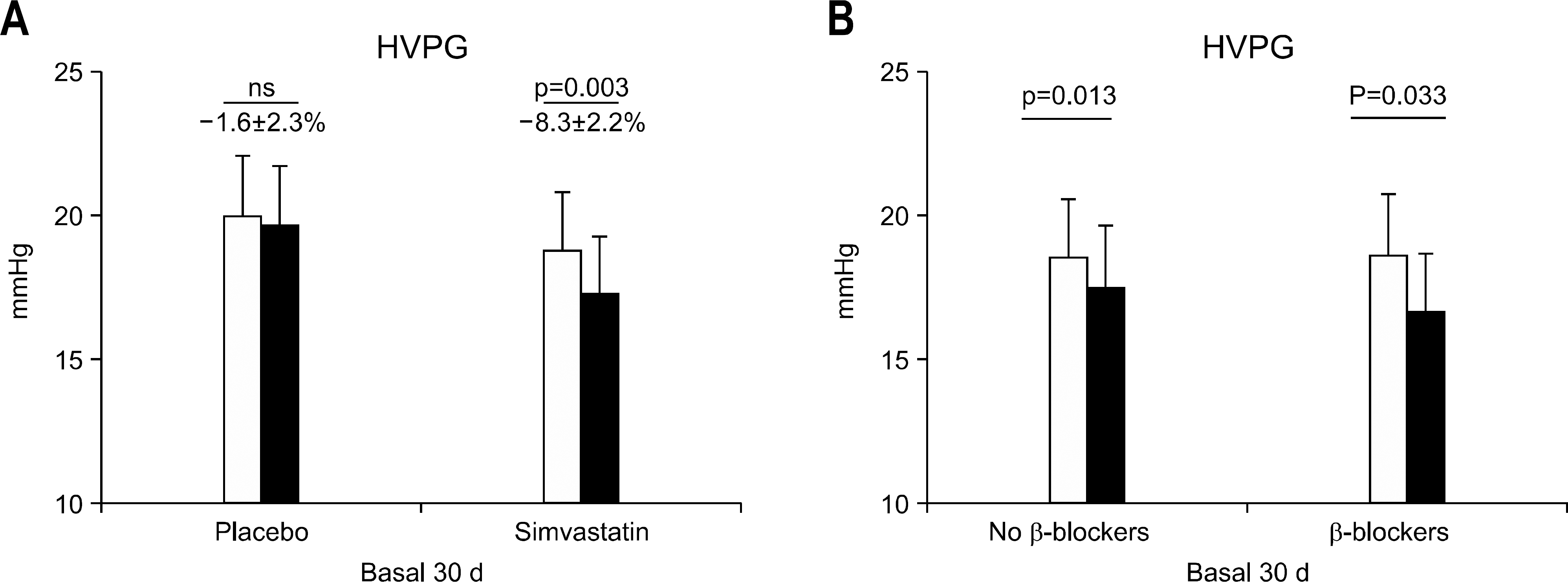
Fig. 4.
Tetrahydropiopterin (BH4) is an essential cofactor for eNOS. Decreased BH4 levels in the cirrhotic liver determine eNOS uncoupling, which results in decreased NO synthesis and increased release of superoxide radicals, which in turn can react with NO, further decreasing the bioavailability of NO. NO, nitric oxide; eNOS, endothelial nitric oxide synthase.
Fig. 5.
Percentage change in HVPG divided by Child Pugh status using individual patient data from three ARB/ACEi versus BB studies. As calculated from individual patient data, the HVPG reduction in patients with Child Pugh A cirrhosis receiving ARB/ACEi's (−17%) was similar to those with Child Pugh A cirrhosis receiving beta-blockade (−21%) and greater than those patients with Child Pugh B/C cirrhosis receiving ARB/ACEi's (3%). The mean percentage change and the 95% confidence limits are represented. (modified from Tandon et al., J of Hepatology 2010). HVPG, hepatic venous pressure gradient; ARB, Angiotensin receptor blocker; ACEi, Angiotensin converting enzyme inhibitor; BB, beta blocker.
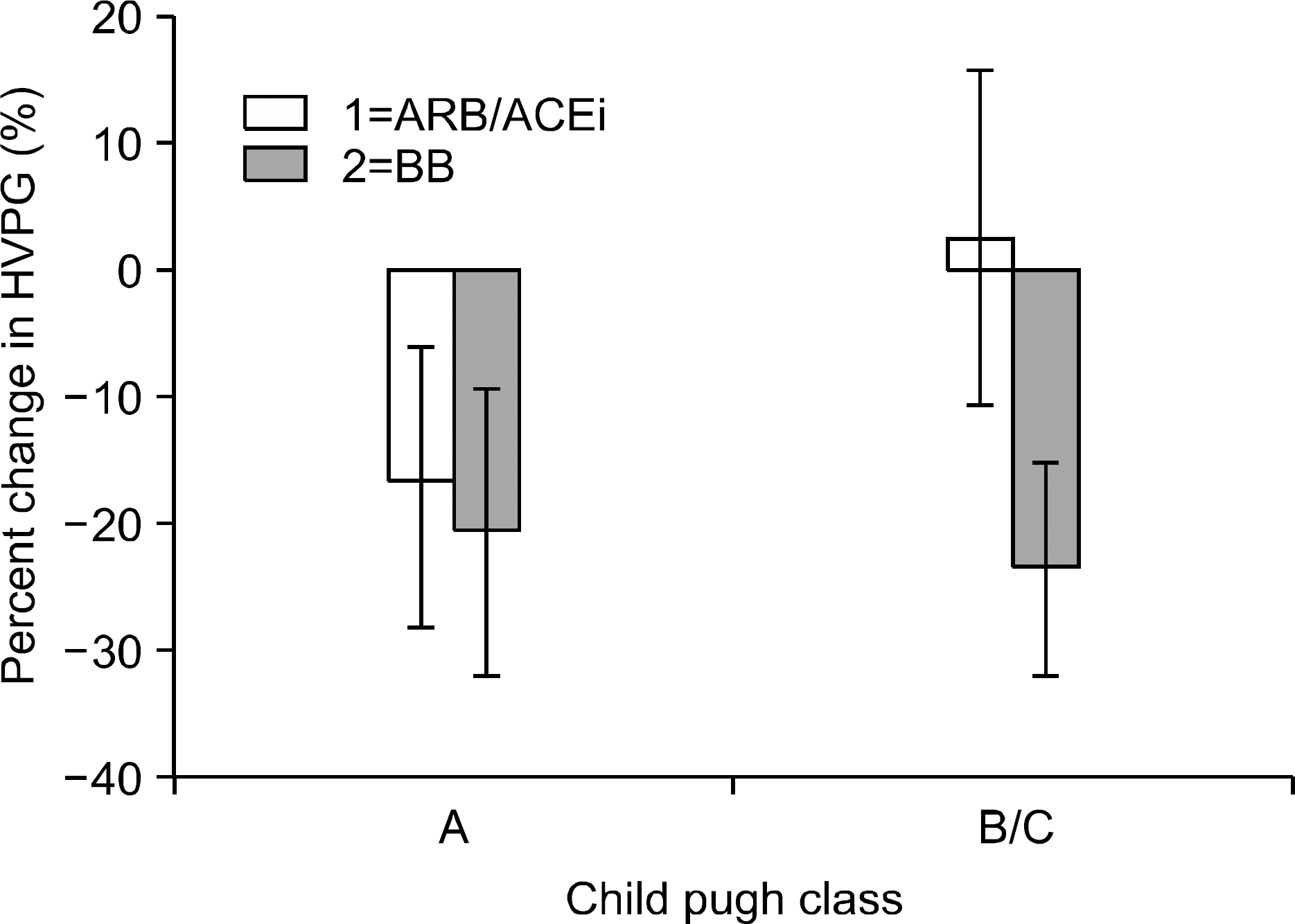
Table 1.
Mechanism for Decreased Hepatic NO Synthesis in Cirrhosis and Possible Treatments
Table 2.
Drugs Used to Reduce Portal Pressure in Cirrhosis and Their Dosage




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download